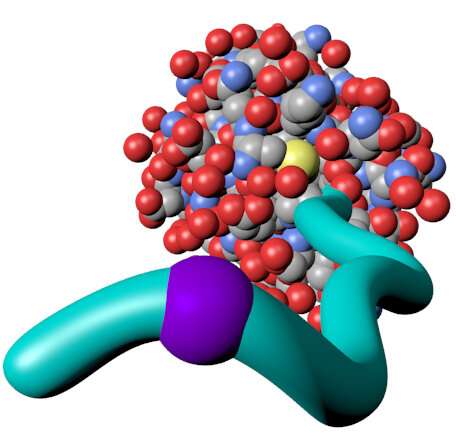
A technique developed by Miami University associate professors of chemistry and biochemistry Dominik Konkolewicz and Rick Page may help enable more rapid and efficient development of new materials for use in pharmaceuticals, biofuels, and other applications.
Konkolewicz's and Page's technique uses nuclear magnetic resonance (NMR) technology to illuminate how proteins and synthetic polymers interact in chemical substances known as bioconjugates.
Why bioconjugates are useful
Proteins can be used to catalyze chemical reactions that are useful in many applications. For example, protein enzymes are used to produce high-fructose corn syrup and insulin is used to treat diabetes. But some proteins are active for only a very short time or they break down easily, so it's just not practical—or cost-effective—to use them. Protein bioconjugates overcome proteins' limitations by attaching synthetic molecules, often polymers, to the protein.
"Proteins have fantastic performance," Konkolewicz says, "but there's not a lot of flexibility in the chemistry we can put into a protein. Polymers offer a huge diversity of structure and function that we can incorporate in to extend the life of the protein or enhance its ability to withstand extreme conditions."
Already there is some commercial development of bioconjugates, such as antibody-drug conjugates used to treat cancer, although the guidelines for how to improve the performance of these substances remains elusive.
Developing new, useful bioconjugates is often difficult and expensive because the process traditionally relies on trial and error: scientists throw a lot of polymer candidates against a proverbial wall of proteins to see what "sticks" in the form of enhanced performance. But just as it doesn't make sense to throw a tennis ball at a Sheetrocked wall expecting it to stick, it doesn't make sense to throw certain polymers at certain proteins expecting them to stick.
Accelerating development through rational design
We understand the nature of tennis balls and drywall well enough to know that "sticking" is not a possible outcome of their interaction, but Page says that scientists don't always understand the nature of proteins and polymers well enough to make similar predictions when it comes to bioconjugation.
"In many cases, we know the structure of the protein, but we don't know the structure of the polymer. We don't know what shape it is, where it attaches to the protein, or how it wraps around or interacts with the protein," Page says.
What's needed, Konkolewicz and Page say, is a set of rules that would enable rational design of new bioconjugates. Such rules would allow chemists to look at the structure of a target protein and design a polymer molecule of the right size, shape, and function to fit it specifically.
"It would be great to be able to say, 'Okay, here's the protein I have. Here are the ways I need to stabilize it, and here are the sorts of polymers we can use for that,'" Page says.
The technique Page and Konkolewicz have developed is the first step in enabling the establishment of such a set of rules.
While previous techniques for examining interactions between proteins and polymers in bioconjugates relied on, for instance, neutron beams—very expensive equipment available at a limited number of facilities around the world—the Miami chemists' technique uses readily available nuclear magnetic resonance (NMR) technology. The key to the technique is placing reporting groups on the synthetic polymers. These reporting groups act something like beacons, allowing researchers to see how close a polymer is to a protein, when the bioconjugate is in an NMR instrument.
The accessibility of NMR technology is important because it vastly increases the capacity of the research community to make discoveries.
"We can't look at every relevant protein ourselves," Konkolewicz says. "We'd have to live for 500 years to do that. By making it accessible, we allow other groups to examine their proteins of interest—catalytic proteins, like our lab focuses on, or therapeutic proteins, or whatever type they study. This technique provides scale."
A breakthrough made possible by Miami's unique environment
Fundamentally, Konkolewicz and Page's technique enables chemists from around the globe to collaborate on the establishment of a set of design rules to guide more rapid development of bioconjugates that are both effective and affordable for use in industrial applications, including pharmaceuticals and biofuels. That's a fitting outcome for a research effort that was itself born out of collaboration.
It's been historically uncommon for scientists from different subfields to team up as Konkolewicz, a synthetic chemist, and Page, a biochemist, have. Konkolewicz and Page say their advance owes to the fact that Miami University fosters collaboration and encourages exploration across a broad range of expertise.
"The environment that we have here at Miami, and the ability and encouragement for groups to collaborate with each other here, has really set us up in the right environment to come up with this breakthrough technique," Page says.
Another aspect of Miami's unique environment is the deep involvement of undergraduate students in research. Four undergraduate students from Konkolewicz's and Page's labs were named as authors of an article reporting on their technique, which was recently published in the open-access flagship Royal Society of Chemistry journal Chemical Science.
Explore further
Citation: Miami chemists' breakthrough technique enables design at the interface of chemistry and biology (2020, July 21) retrieved 21 July 2020 from https://ift.tt/30zZ76y
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
"interface" - Google News
July 21, 2020 at 08:27PM
https://ift.tt/30zZ76y
Miami chemists' breakthrough technique enables design at the interface of chemistry and biology - Phys.org
"interface" - Google News
https://ift.tt/2z6joXy
https://ift.tt/2KUD1V2
Bagikan Berita Ini














0 Response to "Miami chemists' breakthrough technique enables design at the interface of chemistry and biology - Phys.org"
Post a Comment