Abstract
Grafting is possible in both animals and plants. Although in animals the process requires surgery and is often associated with rejection of non-self, in plants grafting is widespread, and has been used since antiquity for crop improvement1. However, in the monocotyledons, which represent the second largest group of terrestrial plants and include many staple crops, the absence of vascular cambium is thought to preclude grafting2. Here we show that the embryonic hypocotyl allows intra- and inter-specific grafting in all three monocotyledon groups: the commelinids, lilioids and alismatids. We show functional graft unions through histology, application of exogenous fluorescent dyes, complementation assays for movement of endogenous hormones, and growth of plants to maturity. Expression profiling identifies genes that unify the molecular response associated with grafting in monocotyledons and dicotyledons, but also gene families that have not previously been associated with tissue union. Fusion of susceptible wheat scions to oat rootstocks confers resistance to the soil-borne pathogen Gaeumannomyces graminis. Collectively, these data overturn the consensus that monocotyledons cannot form graft unions, and identify the hypocotyl (mesocotyl in grasses) as a meristematic tissue that allows this process. We conclude that graft compatibility is a shared ability among seed-bearing plants.
This is a preview of subscription content
Access options
Subscribe to Journal
Get full journal access for 1 year
199,00 €
only 3,90 € per issue
Tax calculation will be finalised during checkout.
Rent or Buy article
Get time limited or full article access on ReadCube.
from$8.99
All prices are NET prices.

Data availability
All data are available in the manuscript, Extended Data and supplementary materials. Deep sequencing reads for grafted, non-grafted and wounded rice have been deposited into the NCBI Sequence Read Archive (SRA) with accession number PRJNA734117. Source data and custom coding scripts for plotting have been deposited into the GitHub repository: https://github.com/GregReeves/Reeves2021_MonocotGrafting. Source data are provided with this paper.
Code availability
Source data and custom R scripts used for individual plots and for browsing transcriptome results, as well as source data and Python scripts used for constructing monocotyledon phylogenies have been deposited at https://github.com/GregReeves/Reeves2021_MonocotGrafting. None of the custom code is central to conclusions of the manuscript.
References
- 1.
Mudge, K., Janick, J., Scofield, S. & Goldschmidt, E. E. A history of grafting. Hortic. Rev. 35, 437–493 (2009).
- 2.
Melnyk, C. W. & Meyerowitz, E. M. Plant grafting. Curr. Biol. 25, 183–188 (2015).
- 3.
The Plant List v.1.1. http://www.theplantlist.org/ (2013).
- 4.
Calderini, I. M. Essai d’expériences sur la graffe des graminées [Experimental trials on grafting grasses]. Ann. Sci. Nat. Bot. 1846, 131–133 (1846).
- 5.
Muzik, T. J. & La Rue, C. D. The grafting of large monocotyledonous plants. Science 116, 589–591 (1952).
- 6.
Obolensky, G. Grafting of plant embryos and the use of ultrasonics. Plant Food Hum. Nutr. 7, 273–288 (1960).
- 7.
McCann, M. C. Chimeric plants—the best of both worlds. Science 369, 618–619 (2020).
- 8.
Melnyk, C. W., Schuster, C., Leyser, O. & Meyerowitz, E. M. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr. Biol. 25, 1306–1318 (2015).
- 9.
Notaguchi, M. et al. Cell–cell adhesion in plant grafting is facilitated by β-1,4-glucanases. Science 369, 698–702 (2020).
- 10.
Melnyk, C. W. et al. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc. Natl Acad. Sci. USA. 115, E2447–E2456 (2018).
- 11.
Iwase, A. et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 21, 508–514 (2011).
- 12.
Pitaksaringkarn, W. et al. XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. 80, 604–614 (2014).
- 13.
Asahina, M. et al. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 16128–16132 (2011).
- 14.
Matsuoka, K. et al. Wound-inducible ANAC071 and ANAC096 transcription factors promote cambial cell formation in incised Arabidopsis flowering stems. Commun. Biol. 4, 369 (2021).
- 15.
Wu, Y. et al. Rice transcription factor OsDOF11 modulates sugar transport by promoting expression of Sucrose transporter and SWEET genes. Mol. Plant 11, 833–845 (2018).
- 16.
Zhong, R., Lee, C., Hahighat, M. & Ye, Z.-H. Xylem vessel-specific SND5 and its homologs regulate secondary wall biosynthesis through activating secondary wall NAC binding elements. New Phytol. 4, 1496–1509 (2021).
- 17.
Růžička, K., Ursache, R., Hejátko, J. & Helariutta, Y. Xylem development—from the cradle to the grave. New Phytol. 207, 519–535 (2015).
- 18.
Zhao, B. et al. Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor. BMC Mol. Biol. 10, 29 (2009).
- 19.
Chan, P. L. et al. Early nodulin 93 protein gene: essential for induction of somatic embryogenesis in oil palm. Plant Cell Rep. 39, 1395–1413 (2020).
- 20.
Schulze, S., Schäfer, B. N., Parizotto, E. A., Voinnet, O. & Theres, K. LOST MERISTEMS genes regulate cell differentiation of central zone descendants in Arabidopsis shoot meristems. Plant J. 64, 668–678 (2010).
- 21.
Wang, B., Sang, Y., Song, J., Gao, X. Q. & Zhang, X. Expression of a rice OsARGOS gene in Arabidopsis promotes cell division and expansion and increases organ size. J. Genet. Genomics 36, 31–40 (2009).
- 22.
Lee, S. C., Kim, S. J., Han, S. K., An, G. & Kim, S. R. A gibberellin-stimulated transcript, OsGASR1, controls seedling growth and α-amylase expression in rice. J. Plant Physiol. 214, 116–122 (2017).
- 23.
Ueguchi-Tanaka, M. et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437, 693–698 (2005).
- 24.
Eguchi, S. & Tamura, M. N. Evolutionary timescale of monocots determined by the fossilized birth-death model using a large number of fossil records. Evolution 70, 1136–1144 (2016).
- 25.
Melnyk, C. W. Plant grafting: insights into tissue regeneration. Regeneration 4, 3–14 (2017).
- 26.
Umehara, M. et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200 (2008).
- 27.
Mylona, P. et al. Sad3 and Sad4 are required for saponin biosynthesis and root development in oat. Plant Cell 20, 201–212 (2008).
- 28.
Scarpella, E. & Meijer, A. H. Pattern formation in the vascular system of monocot and dicot plant species. New Phytol. 164, 209–242 (2004).
- 29.
Jura-Morawiec, J., Tulik, M. & Iqbal, M. Lateral meristems responsible for secondary growth of the monocotyledons: a survey of the state of the art. Bot. Rev. 81, 150–161 (2015).
- 30.
Steffens, B. & Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 170, 603–617 (2016).
- 31.
Tillich, H. J. in Monocots: Systematics and Evolution (eds Wilson, K. L. & Morrison, D.A.) 603–228 (CSIRO Publishing, 2000).
- 32.
Burger, W. C. The question of cotyledon homology in angiosperms. Bot. Rev. 64, 356–371 (1998).
- 33.
Strosse, H., Houwe, I. & Panis, B. in Banana Improvement: Cellular, Molecular Biology, and Induced Mutations (eds Mohan Jain, S. & Swennen, R.) 1–12 (Science Publishers, 2004).
- 34.
Melnyk, C. W. in Xylem: Methods and Protocols (eds de Lucas, M. & Etchhells, J. P.) 91–102 (Humana Press, 2017).
- 35.
Hollins, T. W., Scott, P. R. & Gregory, R. S. The relative resistance of wheat, rye and triticale to take‐all caused by Gaeumannomyces graminis. Plant Pathol. 35, 93–100 (1986).
- 36.
Osbourn, A. E., Clarke, B. R., Lunness, P., Scott, P. R. & Daniels, M. J. An oat species lacking avenacin is susceptible to infection by Gaeumannomyces graminis var. tritici. Physiol. Mol. Plant Pathol. 45, 457–467 (1994).
- 37.
Chng, S., Cromey, M. G. & Butler, R. C. Evaluation of the susceptibility of various grass species to Gaeumannomyces graminis var. tritici. N. Z. Plant Prot. 58, 261–267 (2005).
- 38.
Löytynoja, A. & Goldman, N. Phylogeny-aware gap placement prevents errors in sequence alignment and evolutionary analysis. Science 320, 1632–1635 (2008).
- 39.
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
- 40.
Brown, J. W., Walker, J. F. & Smith, S. A. Phyx: phylogenetic tools for unix. Bioinformatics 33, 1886–1888 (2017).
- 41.
Kozlov, A. M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455 (2019).
- 42.
Smith, S. A. & O’Meara, B. C. TreePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28, 2689–2690 (2012).
- 43.
Hedges, S. B., Dudley, J. & Kumar, S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 22, 2971–2972 (2006).
- 44.
Iwata, N. & Omura, T. Linkage analysis by reciprocal translocation method in rice plants (Oryza sativa L.) I. Linkage groups corresponding to the chromosome 1, 2, 3 and 4. Jpn. J. Breed. 21, 19–28 (1971).
Acknowledgements
We thank A. Greenland and E. Wallington for hosting G.R. at the National Institute of Agricultural Botany; F. Gallo and K. Müller for generating electron microscopy images; U. Paszkowski for providing ccd8 mutant rice; S. Holdgate for providing a culture of G. graminis var. tritici; M. Castle for statistical advice; M. Tester for supplying tetraploid wheat; R. Bates, R. Donald and K. Billakurthi for assistance; M. J. Grangé-Guermente, O. Murshudova and N. Elina for translating foreign language references into English; and D. C. Baulcombe and A. M. Jones for providing feedback on this manuscript. G.R. was supported by a Gates Cambridge Trust PhD Student Fellowship; G.R. and P.S. were supported by European Research Council (ERC) Grant 694733 Revolution and BB/P003117/1 awarded to J.M.H.; G.R., A.T. and M.R.W.J. were supported by a Ceres Agri-Tech Fund award; A.K.N. and C.W.M. were supported by a Wallenberg Academy Fellowship (KAW 2016.0274); and C.M. and C.W.M. were supported by an ERC Starting Grant (GRASP-805094).
Author information
Affiliations
Contributions
G.R. and J.M.H. conceptualized the study. G.R. A.T., P.S., M.R.W.J., A.K.N. and C.M. performed grafting experiments. A.T. and P.S. prepared sequencing samples and uploaded data to NCBI. G.R., A.T., M.R.W.J., A.K.N. and C.M. performed vascular connectivity assays. G.R. analysed data. M.C. and S.B. generated the transgenic GUS-reporter wheat line. M.C., S.B., A.R.B., P.S., C.W.M. and J.M.H. provided advice on experiments. J.F.W. generated phylogenetic trees. The study was supervised by A.R.B., C.W.M. and J.M.H. The manuscript was written by G.R. and J.M.H. with contributions from the other authors. All authors discussed the results, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Cambridge Enterprise has filed a patent with international application no. PCT/GB2019/053232 and publication no. WO/2020/099879, titled ‘Perennial monocotyledon grafting’ and published on 22 May 2020, which includes methods for grafting monocotyledons described in this manuscript. G.R. and J.M.H. are co-inventors on this patent. The other authors declare no competing interests.
Peer review information
Nature thanks Makoto Matsuoka, Colin Turnbull and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Discovery of a method that allows grafting of monocotyledons.
Immature embryos of wheat (T. aestivum - orange) and pearl millet (P. glaucum - blue) were grown in tissue culture to regenerate a fused plant which may simulate grafting. a-d, Grafted plants were not generated after simulating grafting by placing halved calli in contact (a), pressing two scutella together in media (b), slicing scutellar tissue and placing into close contact (c), or placing callus into a toroidal arrangement (d). However, after removal and exchange of the central part of immature wheat and pearl millet embryos, some germinated into what appeared to be vestigial grafted plants (e). Photographs of tissue (left) are next to graphical representations (right). White arrowheads indicate areas of fusion. Scale-bar represents 0.5 cm and applies to all photographs.
Extended Data Fig. 2 Intra-specific wheat grafts form between different genoypes.
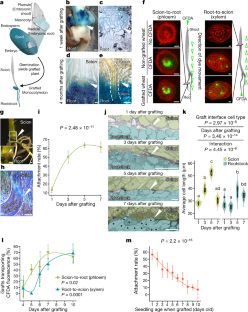
a, Non-grafted wheat seven days after germination. b, Section through the root-shoot interface with the vasculature surrounded by green cells. c, Self-grafted wheat seven days after plumule transplantation. d, Section though graft junction, with the union interrupting the file of green cells linking shoot and root. Parenchyma and scutellar tissues all were connected. e–h, Seven day old β-glucuronidase (GUS) wheat (var. Fielder) grafted to wild type (WT) (var. Paragon): grafted seedling (e), section through the intra-specific graft junction (f), seedling stained with x-gluc (g), and ultra-thin section through the stained graft junction (h). For c–h, n is quantified in k. i, The graft site of a GUS-WT wheat grafted plant four months after grafting and after setting seed. j, X-gluc stained section through the junction shows continuous vascular strands connecting scion and rootstock between dashed lines. For i, j, n=5. k, Rates of fusion between intra-specific GUS-WT grafts one week after grafting, determined destructively by pulling and sectioning (see Supplementary Video 1 for demonstration). Data are presented as box-and-whisker plots displaying median, interquartile range (boxes) and minima and maxima (whiskers) of pooled data from eleven replicate experiments. Comparisons made by two-tailed Fisher’s exact test. l–s, Vascular connectivity in intra-specific wheat grafts. l, p, n, r, The shoot or roots of non-grafted wheat seedlings were inoculated with CFDA-containing agar (l, p) or a mock agar (n and r) solutions seven days after germination as controls to observe vascular transport of fluorescence shoot-to-root (l) or root-to-shoot (p) 1 h following application. m, q, o, s, Even after just 1 h following application, CFDA solutions applied to the shoot or roots of intra-specific GUS-WT wheat grafts identified unsuccessful (o, s) or successful shoot-to-root (m) and root-to-shoot (q) vascular connectivity as shown by empty green arrowheads. GUS-WT grafts successfully transporting CFDA across the graft junction were stained with x-gluc and are shown to the right of the CFDA channel (m, q). t–v, Root cross-sections of the CFDA phloem connection assay seven days after grafting on intra-specific GUS-WT wheat grafts from l, m, and n, respectively. w–y, Shoot cross-sections of the CFDA phloem connection assay seven days after grafting. Propidium iodide (PI) was applied on the cross-sections (t–y), and images were acquired by fluorescence microscopy (l–y). Negative controls (n, r, t, w) showed very little autofluorescence. For l–y, n=10 plants evaluated for CFDA movement in grafts and non-graft controls. White arrowheads indicate the graft junction (c–j, m, q, o, s). BF, bright field channel. Scale bars represent 1 mm (a, c, e, g, i), 500 μm (l-s), or 100 μm (b, d, f, h, j, t–y).
Extended Data Fig. 3 Grafted rice forms functional vascular connections.
a–d, Bright-field (BF) images of toluidine blue (TB) or sodium hydroxide (OH-) cleared mesocotyl tissue in non-grafted (a, b) or grafted (c, d) rice seven days after grafting. The vascular cylinder is shown between dashed lines. For a, b, n=20, and for c, d, n=65. e–l, Representative BF and confocal microscopy fluorescence images for exogenous CFDA dye application indicate movement across graft junctions from shoot-to-root and root-to-shoot in the vasculature. Non-grafted plants transport CFDA from shoot-to-root (e) and from root-to-shoot (i). Mock solutions generated no CFDA fluorescence signal (g, k). Grafted plants transport CFDA from shoot-to-root (f) and from root-to-shoot (j). Lack of CFDA transport is indicative of failed or delayed graft formation (h, l). m–r, Cross-sections indicating CFDA transport to the rootstock (plants from e, f, and h) or scion (plants from i, j, and l) after application of CFDA to the scion or rootstock, respectively, seven days after grafting (grafted five days after germination). All sections were stained with propidium iodide (PI) to visualize shoot or root structure. Images from the CFDA, PI and merged channels are presented. No CFDA fluorescence was detectable in shoots after application of mock agar (m). However, when CFDA was provided to roots of non-grafted (n) and grafted plants (o), signal was detected surrounding xylem tissues of the shoot. Merged images show that CFDA is localized to vascular strands. Enlarged panels show xylem vessels. No CFDA fluorescence was detectable in roots after application of mock agar (p), however, when CFDA was provided to shoots of non-grafted (q) and grafted plants (r), signal was detected in vascular tissue of the root. Merged images show that CFDA is localized to vascular strands. All images acquired by confocal microscopy. s, Attachment rate of rice grafts over time (plants from e-r). n=14, 15, 10, 20, 20, 40, 20, 14, 20 (non-grafted control), n=30, 30, 60, 50, 59, 240, 120, 40, 80 (grafted) (1, 2, 3, 4, 5, 6, 7, 10, 14 days after grafting, respectively). t, CFDA transport rates in attached grafts from s show vascular connectivity increases over the course of seven days. Data are presented as mean grafting rate ± s.d. of three to six replicate experiments (blocking/random effect). n=29, 30, 38, 18, 14. (phloem, non-grafted control) n=26, 28, 29, 18, 13 (xylem, non-grafted control), n=8, 14, 45, 20, 12 (phloem, grafted), n=9, 11, 31, 30, 11 (xylem, grafted) (4,5,6,7,10 days after grafting, respectively). Comparisons were made using mixed effect binomial regression with replicate experiments as a random effect (s, t). White arrowheads indicate graft junctions (c, d, f, h, j, and l). Scale bars represent 1 mm (e–l), 100 µm (a–d), or 50 µm (m–r).
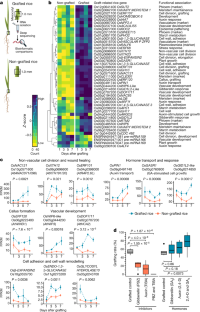
Extended Data Fig. 4 Overview of changes in transcript abundance during the development of graft unions in rice.
a, Diagram of non-grafted, wounded, and grafted rice (var. Kitaake) tissue harvested for transcriptome analysis one, three, five, and seven days after grafting. b, Three dimensional priniciple component analysis (PCA) of individual biological replicates for non-grafted, wounded, and grafted rice. n=3. c, Upset plot for differentially expressed genes (DEGs) for grafted versus non-grafted, and grafted versus wounded rice. d, The fifteen most up-regulated GO terms in grafted compared with wounded rice. See Supplementary Table 4 for complete GO term information. e, Transcriptional dynamics of genes associated with graft formation. Data are presented as mean ± s.e.m, and as wounded gene expression overlaps significantly with grafted gene expression during dicotyledon grafting10, comparisons were made between grafted rice versus non-grafted rice expression by two-way ANOVA. n=3. Apart from cambium-maker genes, there was significant overlap of gene expression in rice compared to those associated with dicotyledon grafting.
Extended Data Fig. 5 Dynamics of transcripts from cell expansion and cell division genes during graft formation in rice.
a, The fifteen most up-regulated GO terms relative to each adjacent timepoint. Full list of GO details in Supplementary Table 5. b, Z-scores for differentially expressed transcripts of cell expansion genes in the small auxin up regulated (SAUR), EXPANSIN and GLYCOSYLHYDROSE families (left), as well as for cell cycle genes (right) in grafted rice over time. Gene details in Supplementary Table 6.
Extended Data Fig. 6 Specific gene family analysis for rice graft transcriptomics.
a, Z-score normalized transcription factors with differential transcript abundance within the APETALA2/ETHYLENE-RESPONSIVE FACTOR (AP2/ERF); NO APICAL MERISTEM, ARABIDOPSIS TRANSCRIPTION ACTIVATION FACTOR, CUP SHAPED COTYLEDON (NAC); MYELOBLASTOSIS (MYB); DNA-BINDING ONE FINGER (DOF); and AUXIN RESPONSE FACTOR (ARF) families between non-grafted and grafted rice over time as determined by significant Two-way ANOVA grouping variable (graft type, P < 0.05). Gene details in Supplementary Table 7. b, Pre-microRNA transcripts showing reduced expression during grafting and their potential targets involved in plant development. c, Early nodulin genes expression during grafting in rice (above) and Arabidopsis during grafting. Arabidopsis data was adapted from Melnyk et al. 201810. Data are presented as mean ± s.e.m, and comparisons made by two-way ANOVA (grafted rice versus non-grafted expression) (b, c). n=3.
Extended Data Fig. 7 Grafting across all three monocotyledonous groups.
a, b, Representative images of non-grafted seedlings (a), and hand cross sections of plant tissue (b) stained with toluidine blue representing species from Alismatid, Lilioid, and Commelinid monocotyledons. c, d, Representative images of grafted seedlings seven to sixty days after grafting (c), and hand cross sections through the graft junction stained with toluidine blue (d). Panels in the upper right corner show higher magnification of the graft site (c). Non-grafted controls are the same age as grafted plants. For a, b, n=5 and for c, d, n=2 (Ananas comosus), n=8 (Costus laevis), n=18 (Commelina comminis), n=8 (Beaucarnea recurvata), n=16 (Dioscorea elephantipes), n=12 (Gloriosa superba), n=12 (Arisaema tortuosum), n=39 (Acorus calamus). White arrowheads indicate the graft junctions (c, d). Scale-bars represent 1 mm (a, c) or 200 µm (b, d).
Extended Data Fig. 8 Intra- and inter-specific graft combinations in the Poaceae.
a, Phylogenetic reconstruction of the twelve subfamilies comprising the Poaceae (grass family). Species grafted are shown in bold. b, Rates of fusion between different varieties of the same C3 or C4 photosynthetic cereal crop species. c, Rates of fusion between different C3 cereal species.
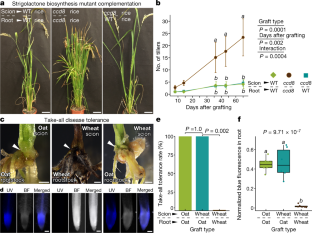
Extended Data Fig. 9 Complementation of rice defective in strigolactone biosynthesis by grafting mutants onto wild-type rootstocks.
a, Position of the carotenoid cleavage dioxygenase 8 (OsCCD8, Os01g0746400) gene in the rice strigolactone biosynthesis pathway. Red color indicates the homozygous mutant ccd8/ccd8 allele (the d10 mutant44). b, Height of the tallest ligule (upper plot), and length of the longest leaf (lower plot) on each grafted plant measured over time. Statistically significant differences for tallest ligule height and longest leaf length were assessed by two-way repeated measures ANOVA among graft types over time. Letters indicate significant (P < 0.05) groupings among graft types at each time point using pairwise t-tests with adjusted P values by the Bonferroni multiple testing correction method. ns = not significant. Data are presented as mean ± s.d. n=8. c, Shoot phenotypes of initial grafts (upper panel) and their offspring derived from self-pollination (lower panel) after 40 days of growth in soil. d, Rates of reversion to a high tillering (mutant) phenotype in the offspring of self-pollinated grafts between ccd8 mutant (d10) and wild type (O. sativa var. Shiokari). Although the mutant phenotype was rescued when grafted to a wild type root system, offspring of the mutant scions from such grafts reverted to the mutant phenotype, as expected. Scale-bars represent 10 cm (c).
Extended Data Fig. 10 Grafting wheat to oat confers disease tolerance to take-all.
a, A schematic used for screening wheat, oat, and inter-tribal grafts between wheat and oat for take-all disease, caused by the soil borne pathogen G. graminis var. tritici. b, Representative images of self-grafted oat, self-grafted wheat, and inter-tribal grafts between wheat and an oat. Transverse sections of each graft junction are shown in the upper right corner of each panel seven days after grafting. n=6. c, Representative images of grafted plants seven days after grafting and immediately after inoculation with an agar plug containing G. graminis var. tritici (n = 6). The plug was placed directly on top of the roots to ensure physical contact between the pathogen and rootstock. d, Representative images of grafted plants three weeks after inoculation. Panels show high magnification of graft junction (left), side view of the culture containers (middle), and a view looking down into the containers (right). e, Non-inoculated control grafts, with side view of culture containers (left) and view looking down into the container (right). White arrowheads indicate the graft junction (a–e), and empty black arrowheads indicate disease progression into the scion past the graft junction (d). Scale bars represent approximately 1 mm (b, d left panels), 250 µm (b upper right panels), 1 cm (c, d middle and right, and e).
Supplementary information
Supplementary Information
This file contains Supplementary Note 1 (Graft compatibility within monocotyledons) and Supplementary Note 2 (Historical reports of monocotyledon grafting), plus additional references.
Supplementary Table 1
Spreadsheet containing total numbers and rates for grafting all monocotyledons in this study. Individual sheets are labelled according to the source data used in each figure of this article.
Supplementary Table 2
Spreadsheet of FPKM values for all genes expressed in across the replicates of grafted, non-grafted, or wounded rice at one, three, five and seven days after grafting. Plots for any gene of interest can be displayed. Up to four plots can be displayed side-by-side for visual comparisons.
Supplementary Table 3
Spreadsheet of all differentially expressed genes between grafted and non-grafted rice, or grafted and wounded rice. Each sheet is separated according to each time point and up- or downregulated genes.
Supplementary Table 4
Spreadsheet of all statistically significant Gene Ontology (GO) terms between grafted and non-grafted rice, or grafted and wounded rice. Each sheet is separated according to each time point and up- or downregulated GO terms.
Supplementary Table 5
Spreadsheet of all statistically significant Gene Ontology (GO) terms between grafted rice at adjacent timepoints during graft union formation partly shown in the bar plots of Extended Data Fig. 5a. Each sheet is separated according to each time point and up- or downregulated GO terms.
Supplementary Table 6
Spreadsheet with gene ID information for the transcript abundance of cell expansion, cell elongation, cell cycle and cell growth regulators in grafted rice shown in the heat map plots of Extended Data Fig. 5b.
Supplementary Table 7
Spreadsheet with gene ID information for the transcript abundance of differentially expressed genes within the AP2/ERF, NAC, MYB, DOF and ARF transcription factor families between grafted and non-grafted rice shown in the heat map plots of Extended Data Fig. 6a.
Supplementary Table 8
Germination and growth protocols for monocotyledonous species grafted in Fig. 3b.
Supplementary Video 1
Attempted mechanical separation and dissection of a grafted wheat seedling. Real-time footage shows two pairs of forceps attempting to pull apart a grafted intra-specific wheat seedling on wet filter paper one week after grafting. No adhesive or physical attachment were used to aid grafting. The junction did not separate when force was applied in opposing directions from the rootstock and scion. The junction was then bisected laterally with a razor blade to show the fused tissue. This procedure was used to evaluate grafts throughout this study. The video footage was captured at 10× magnification with an iPhone SE mounted to a Zeiss Stemi 508 stereomicroscope and was edited using Blender (v.2.80).
Supplementary Video 2
Intra-specific cereal grafting. A demonstration of embryonic shoot (plumule) and embryonic root (radicle) microdissection used to graft different individuals within five cereal species. Following this procedure, seeds are germinated into grafted plants. The video footage was captured on a Leica S8 APO stereomicroscope (Milton Keynes, UK) with a GT Vision GXCAM HICHROME-MET High Resolution Camera (Sudbury, UK) at 10× magnification, sped up 2.5 times, and was edited in Blender (v.2.80).
Supplementary Video 3
Inter-specific cereal grafting. A demonstration of embryonic shoot (plumule) and embryonic root (radicle) microdissection used to graft different individuals among five cereal species. Following this procedure, seeds are germinated into grafted plants. The video footage was captured on a Leica S8 APO stereomicroscope (Milton Keynes, UK) with a GT Vision GXCAM HICHROME-MET High Resolution Camera (Sudbury, UK) at 10× magnification, sped up 2.5 times, and was edited in Blender (v.2.80).
Source data
Rights and permissions
About this article
Cite this article
Reeves, G., Tripathi, A., Singh, P. et al. Monocotyledonous plants graft at the embryonic root–shoot interface. Nature (2021). https://ift.tt/3H8ttku
-
Received:
-
Accepted:
-
Published:
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
"interface" - Google News
December 22, 2021 at 11:01PM
https://ift.tt/3piVYFK
Monocotyledonous plants graft at the embryonic root–shoot interface - Nature.com
"interface" - Google News
https://ift.tt/2z6joXy
https://ift.tt/2KUD1V2
Bagikan Berita Ini














0 Response to "Monocotyledonous plants graft at the embryonic root–shoot interface - Nature.com"
Post a Comment