Many people consider battery-powered electronic devices such as cell phones, computers, and even electric vehicles essential. On the other hand, these gadgets usually involve charging at least once a day. Developing batteries that can store additional energy are required to extend the time between charges.
While lithium metal electrodes promise to increase energy density, their instability implies that the batteries have a limited service life and are connected with substantial safety risks.
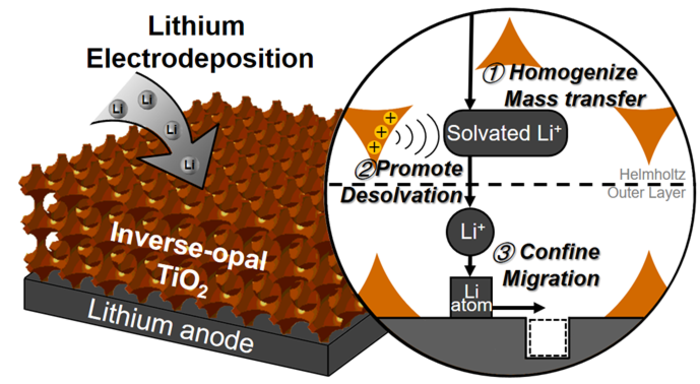
A pair of researchers from Nankai University in China and the University of Cambridge in the United Kingdom demonstrate a new approach for stabilizing lithium electrodes in a paper published in the KeAi journal Green Energy & Environment. It uses an inverse-opal structured electrode interface protection layer to efficiently manage ion electrodeposition on the electrode surface.
Lithium is just like an unruly child. Its capricious behavior in the electrode reaction process leads to an uneven electrode surface and sharp dendrites that can pierce the separator and cause a fire. These make the battery unstable and unsafe.
Guoran Li, Study Author and Professor, Materials Chemistry, School of Materials Science and Engineering, Nankai University
Prof. Li and their research colleagues chose to abandon the usual approach of protecting electrode surfaces from corrosion to develop a membrane with a linear structure and active components to manage lithium behavior.
The highly ordered channels in the inverse-opal structure even out the lithium ion distribution, and effectively regulate every stage of the electrodeposition process to achieve the final target, i.e., make the lithium metal electrode work stably for the batteries.
Guoran Li, Study Author and Professor, Materials Chemistry, School of Materials Science and Engineering, Nankai University
This is a noteworthy discovery in the lithium (Li) metal electrode field, according to Wu Xuewen, the PhD student who headed the investigation and data curation.
Our research shows that the regular structure and active components of the protective membrane can effectively regulate the electrode reaction process to improve the final electrochemical performance. We hope that our study can provide a new perspective for the detailed exploration of the reaction process of Li metal electrodes, and promote the practical application of high-performance Li metal batteries.
Guoran Li, Study Author and Professor, Materials Chemistry, School of Materials Science and Engineering, Nankai University
Journal Reference:
Wu, X., et al. (2022) Inverse-opal structured TiO2 regulating electrodeposition behavior to enable stable lithium metal electrodes. Green Energy & Environment. doi.org/10.1016/j.gee.2022.03.010.
Source: https://www.keaipublishing.com/en/
"interface" - Google News
May 20, 2022 at 08:51PM
https://ift.tt/DJ3CH5U
Researchers Develop an Inverse-Opal Electrode Interface Protective Film - AZoM
"interface" - Google News
https://ift.tt/7bX9zWZ
https://ift.tt/OnDBvsu
Bagikan Berita Ini














0 Response to "Researchers Develop an Inverse-Opal Electrode Interface Protective Film - AZoM"
Post a Comment