Graphene is a 2D material where carbon atoms are organized in hexagonal structures. This material has special chemical and physical properties, like thermal and electrical conductivity, mechanical flexibility, chemical stability, selective permeability to water, sub-nanometer thickness, and optical transparency.
Because of these properties, many different applications of graphene within catalysts, electrical energy storage, desalination, and transparent electrodes have been extensively analyzed.
Graphene is an extremely thin material and, hence, to make it viable for practical applications, it needs to be deposited over other materials that act as substrate.
One of the topics which are of significant scientific interest is how intercalations take place between water and graphene on a substrate. Wettability is the potential of the interfacial water to retain contact with a solid surface, and it relies on the hydrophobicity of a material. The wettability of graphene is different from a majority of materials and it varies based on the kind of substrate.
To be more specific, the wettability of the substrate is not strongly influenced by the presence of one layer of graphene on its surface. This unusual wettability of graphene has been explained by the term “wetting transparency” because the wetting characteristics at the graphene-water interface do not have much impact on the interaction between the substrate and water via the thin graphene.
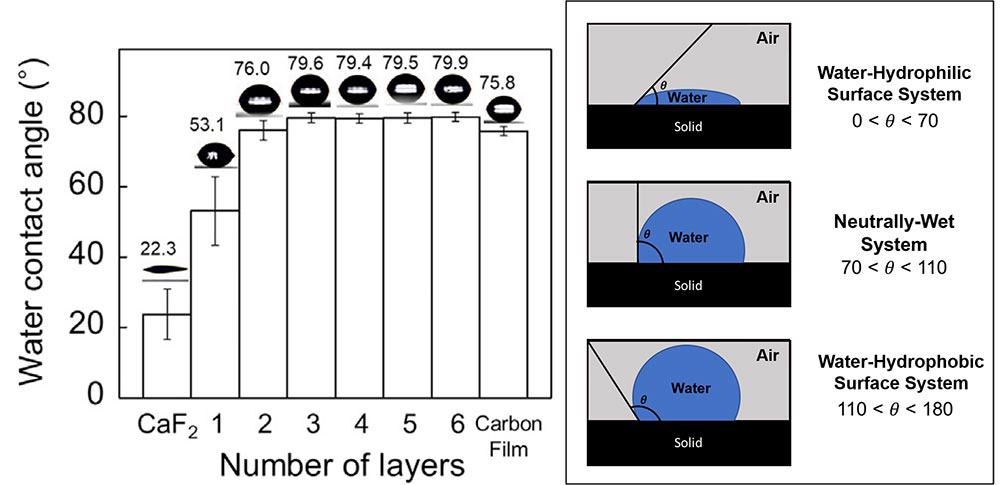
Different water contact angle (WCA) measurements have been made to analyze the wettability of graphene on different kinds of substrates. WCA is a commonly used method to quantify the material hydrophobicity because when the material becomes more hydrophobic, the contact angle between the material and water droplet also increases.
Such analyses have hinted that although the wettability of the graphene monolayer is considerably transparent, the graphene becomes more and more hydrophobic with the increase in the number of layers.
But the WCA measurement can provide only data on the macroscopic characteristics of the interface between the graphene and water, and it cannot provide a complete picture of interfacial water present at the graphene-water interface.
While other methods, like reflection-based infrared spectroscopy or Raman spectroscopy, have been often used for quantifying microscopic properties, they are not suitable for selectively visualizing the interfacial water molecules.
The reason for this is that the vibrational spectroscopic signal of interfacial water molecules is fully concealed by the large signal emitted from bulk water. Consequently, it is no surprise that molecular-level studies have been largely lacking in this field of graphene research.
In the recent past, a team of researchers from the Center for Molecular Spectroscopy and Dynamics (CMSD) within the Institute for Basic Science (IBS) based in Seoul, South Korea, and the Korea University demonstrated the origin of graphene wettability.
Using a method known as “vibrational sum-frequency generation spectroscopy (VSFG),” the researchers effectively visualized the hydrogen-bond structure of water molecules at graphene-water interfaces. As second-order nonlinear spectroscopy, VSFG can be used to selectively examine molecules with damaged centrosymmetry.
VSFG is the perfect technique for analyzing the structures and behavior of water molecules at the graphene interface because within the bulk liquid the water molecules are invisible owing to their isotropic distribution of molecular orientations.
The researchers also noted the VSFG spectra of water molecules on multi-layer graphene enclosing a calcium fluoride (CaF2) substrate. The team was able to monitor the differences in the hydrogen-bond structure of water molecules.
When four or more graphene layers are present, a typical peak at around 3,600 cm−1 began to emerge in the VFSG spectra. Such a peak correlates with the water molecules with the dangling -OH groups that do not create hydrogen bonds with adjacent water molecules, which is a typical trait often found in water at the hydrophobic interface.
The outcome is the first observation demonstrating the hydrogen-bond structure of water molecules at the water-graphene interface.
The team also compared the VSFG wettability value that could be estimated from the quantified spectra to the calculated adhesion energy relevant to the quantified WCAs.
The researchers observed that both characteristics are highly correlated with one another. Such an observation indicates that the VSFG could be a useful tool for analyzing the wettability of 2D materials at the molecular level.
It also demonstrated that the VSFG technique could be used as an alternative for quantifying the adhesion energy of water on hidden surfaces, where quantifying the water contact angle is hard or even impossible.
“This study is the first case describing the increasing hydrophobicity of the graphene surface at a molecular level depending on the number of graphene layers,” and “Vibrational sum-frequency generation spectroscopy could be used as a versatile tool for understanding the properties of any functional two-dimensional materials,” noted the first and second authors of the study KIM Donghwan and KIM Eunchan Kim.
For applications where graphene is utilized in water solution, the hydrophobicity of the interface is one of the key factors in determining the efficiency of graphene layers for various applications. This research is expected to provide basic scientific knowledge for an optimal design of graphene-based devices in the future.
Minhaeng Cho, Professor and Director, CMSD
Journal Reference:
Kim, D., et al. (2021) Wettability of graphene and interfacial water structure. Chem. doi.org/10.1016/j.chempr.2021.03.006.
Source: https://www.ibs.re.kr/
"interface" - Google News
April 22, 2021 at 09:34PM
https://ift.tt/3auxuS4
Study Sheds New Light on Graphene Interface Properties at Microscopic Levels - AZoNano
"interface" - Google News
https://ift.tt/2z6joXy
https://ift.tt/2KUD1V2
Bagikan Berita Ini














0 Response to "Study Sheds New Light on Graphene Interface Properties at Microscopic Levels - AZoNano"
Post a Comment