Study shows eCOA/ePRO interface is equivalent to responses from text-based smartphone formats.
Clinical eCOA/ePRO platform technology provider uMotif has released topline study results demonstrating its proprietary data capture interface incorporated into its eCOA/ePRO app offers a validated alternative to current text-based smartphone-based instruments for clinical, post-marketing and real-world research.
The independent quantitative equivalence study was conducted by SAFIRA Clinical Research at Dublin City University. Fifty-five participants completed testing in two randomized groups using the Motif visual interface and a traditional 5-point Verbal Response Scale (VRS-5). The study results showed equivalence between the Motif interface and standard VRS (high ICC) for capturing VRS data. Study results are expected to be published in 2023.
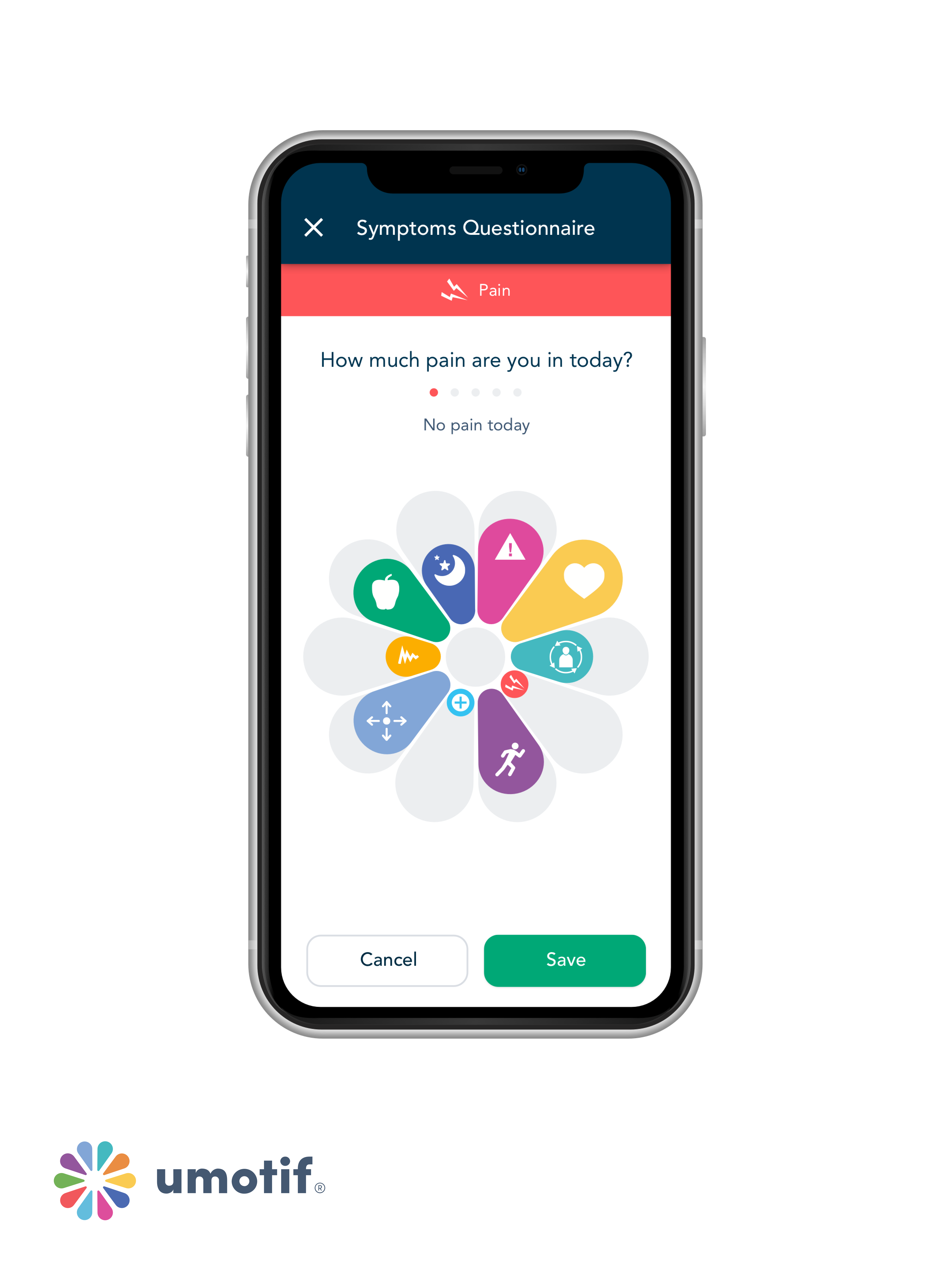
Bruce Hellman, Co-Founder and Chief Patient Officer expanded on the history of eCOA/ePRO to the significance of this study to Applied Clinical Trials. “Traditionally, PROs were paper, with a question at the top of the sheet, and patients would check one of the boxes [answers].” This same format migrated onto PDAs, then smartphones, but in the paper/text/content format. “We knew that patients found our app more engaging…it’s quicker to use, and it’s easier to use for different populations and age groups,” said Hellman. However, eCOA scientists have always asked that PROs could be validated from paper to PDAs, then PDAs to smartphones, now the equivalence of text-form smartphones to graphical user interfaces--the uMotif interface uses a flower-petal visual, with a finger-swipe indicator for responses.
Advertisement
"interface" - Google News
December 07, 2022 at 04:58AM
https://ift.tt/RWdfZml
uMotif Announces Validated eCOA/ePRO Interface - Applied Clinical Trials Online
"interface" - Google News
https://ift.tt/NV602ZC
https://ift.tt/CItFuLU
Bagikan Berita Ini














0 Response to "uMotif Announces Validated eCOA/ePRO Interface - Applied Clinical Trials Online"
Post a Comment