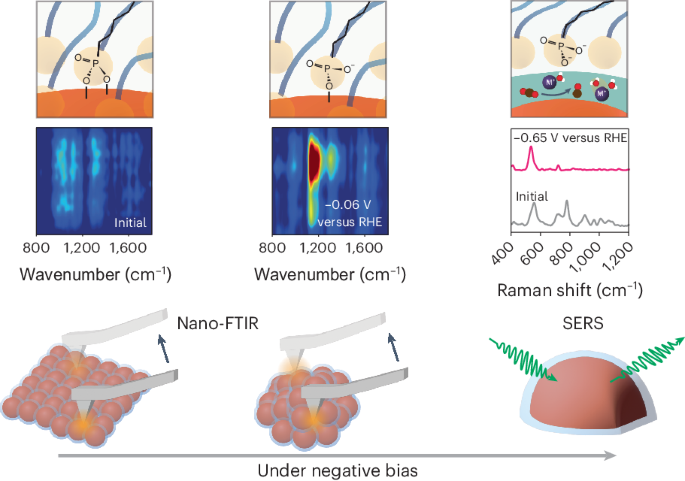
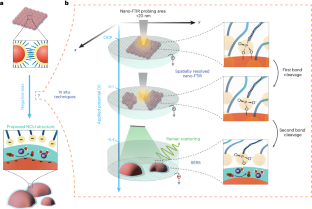
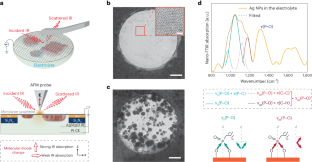
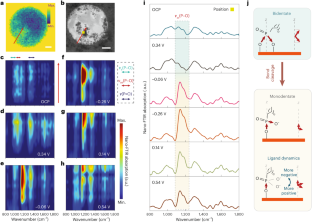
Abstract
The dynamic response of surface ligands on nanoparticles (NPs) to external stimuli critically determines the functionality of NP–ligand systems. For example, in electrocatalysis the collective dissociation of ligands on NP surfaces can lead to the creation of an NP/ordered-ligand interlayer, a microenvironment that is highly active and selective for CO2-to-CO conversion. However, the lack of in situ characterization techniques with high spatial resolution hampers a comprehensive molecular-level understanding of the mechanism of interlayer formation. Here we utilize in situ infrared nanospectroscopy and surface-enhanced Raman spectroscopy, unveiling an electrochemical bias-induced consecutive bond cleavage mechanism of surface ligands leading to formation of the NP/ordered-ligand interlayer. This real-time molecular insight could influence the design of confined localized fields in multiple catalytic systems. Moreover, the demonstrated capability of capturing nanometre-resolved, dynamic molecular-scale events holds promise for the advancement of using controlled local molecular behaviour to achieve desired functionalities across multiple research domains in nanoscience.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
All data are available from the authors on reasonable request.
References
-
Chen, G., Roy, I., Yang, C. & Prasad, P. N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 116, 2826–2885 (2016).
-
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
-
Owen, J. The coordination chemistry of nanocrystal surfaces. Science 347, 615–616 (2015).
-
Kelly, K. L., Coronado, E., Zhao, L. L. & Schatz, G. C. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B 107, 668–677 (2003).
-
Guntern, Y. T. et al. Colloidal nanocrystals as electrocatalysts with tunable activity and selectivity. ACS Catal. 11, 1248–1295 (2021).
-
Xie, C., Niu, Z., Kim, D., Li, M. & Yang, P. Surface and interface control in nanoparticle catalysis. Chem. Rev. 120, 1184–1249 (2020).
-
Puntes, V. F., Krishnan, K. M. & Alivisatos, A. P. Colloidal nanocrystal shape and size control: the case of cobalt. Science 291, 2115–2117 (2001).
-
Sun, Y. & Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002).
-
Le Goas, M. et al. (In)stability of ligands at the surface of inorganic nanoparticles: a forgotten question in nanomedicine?. Nano Today 45, 101516 (2022).
-
Heuer-Jungemann, A. et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 119, 4819–4880 (2019).
-
Rossi, L. M., Fiorio, J. L., Garcia, M. A. S. & Ferraz, C. P. The role and fate of capping ligands in colloidally prepared metal nanoparticle catalysts. Dalton Trans. 47, 5889–5915 (2018).
-
Dabbagh, A. et al. Polyethylene glycol-coated porous magnetic nanoparticles for targeted delivery of chemotherapeutics under magnetic hyperthermia condition. Int. J. Hyperthermia 36, 104–114 (2019).
-
Le Goas, M. et al. Irradiation effects on polymer-grafted gold nanoparticles for cancer therapy. ACS Appl. Bio Mater. 2, 144–154 (2019).
-
Yan, B. et al. Characterization of surface ligands on functionalized magnetic nanoparticles using laser desorption/ionization mass spectrometry (LDI-MS). Nanoscale 5, 5063–5066 (2013).
-
Yu, D., Wehrenberg, B. L., Jha, P., Ma, J. & Guyot-Sionnest, P. Electronic transport of n-type CdSe quantum dot films: effect of film treatment. J. Appl. Phys. 99, 104315 (2006).
-
Lu, L., Zou, S. & Fang, B. The critical impacts of ligands on heterogeneous nanocatalysis: a review. ACS Catal. 11, 6020–6058 (2021).
-
Kim, D. et al. Selective CO2 electrocatalysis at the pseudocapacitive nanoparticle/ordered-ligand interlayer. Nat. Energy 5, 1032–1042 (2020).
-
Yu, S. et al. Nanoparticle assembly induced ligand interactions for enhanced electrocatalytic CO2 conversion. J. Am. Chem. Soc. 143, 19919–19927 (2021).
-
Yu, S., Louisia, S. & Yang, P. The interactive dynamics of nanocatalyst structure and microenvironment during electrochemical CO2 conversion. JACS Au 2, 562–572 (2022).
-
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
-
Bechtel, H. A., Johnson, S. C., Khatib, O., Muller, E. A. & Raschke, M. B. Synchrotron infrared nano-spectroscopy and -imaging. Surf. Sci. Rep. 75, 100493 (2020).
-
Wu, C.-Y. et al. High-spatial-resolution mapping of catalytic reactions on single particles. Nature 541, 511–515 (2017).
-
Bechtel, H. A., Muller, E. A., Olmon, R. L., Martin, M. C. & Raschke, M. B. Ultrabroadband infrared nanospectroscopic imaging. Proc. Natl Acad. Sci. USA 111, 7191–7196 (2014).
-
Muller, E. A., Pollard, B., Bechtel, H. A., Van Blerkom, P. & Raschke, M. B. Infrared vibrational nano-crystallography and nano-imaging. Sci. Adv. 2, e1601006 (2016).
-
Amenabar, I. et al. Structural analysis and mapping of individual protein complexes by infrared nanospectroscopy. Nat. Commun. 4, 2890 (2013).
-
Zhao, X. et al. In vitro investigation of protein assembly by combined microscopy and infrared spectroscopy at the nanometer scale. Proc. Natl Acad. Sci. USA 119, e2200019119 (2022).
-
Lu, Y.-H. et al. Ultrathin free-standing oxide membranes for electron and photon spectroscopy studies of solid–gas and solid–liquid interfaces. Nano Lett. 20, 6364–6371 (2020).
-
Lu, Y.-H. et al. Infrared nanospectroscopy at the graphene–electrolyte interface. Nano Lett. 19, 5388–5393 (2019).
-
He, X., Larson, J. M., Bechtel, H. A. & Kostecki, R. In situ infrared nanospectroscopy of the local processes at the Li/polymer electrolyte interface. Nat. Commun. 13, 1398 (2022).
-
Zenobi, M. C., Luengo, C. V., Avena, M. J. & Rueda, E. H. An ATR-FTIR study of different phosphonic acids in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 70, 270–276 (2008).
-
Luschtinetz, R., Seifert, G., Jaehne, E. & Adler, H.-J. P. Infrared spectra of alkylphosphonic acid bound to aluminium surfaces. Macromol. Symp. 254, 248–253 (2007).
-
Geldof, D. et al. Binding modes of phosphonic acid derivatives adsorbed on TiO2 surfaces: assignments of experimental IR and NMR spectra based on DFT/PBC calculations. Surf. Sci. 655, 31–38 (2017).
-
Lee, J. T., Hati, S., Fahey, M. M., Zaleski, J. M. & Sardar, R. Surface-ligand-controlled enhancement of carrier density in plasmonic tungsten oxide nanocrystals: spectroscopic observation of trap-state passivation via multidentate metal phosphonate bonding. Chem. Mater. 34, 3053–3066 (2022).
-
Klähn, M. et al. IR spectra of phosphate ions in aqueous solution: predictions of a DFT/MM approach compared with observations. J. Phys. Chem. A 108, 6186–6194 (2004).
-
Skibinski, E. S., DeBenedetti, W. J. I. & Hines, M. A. Solution deposition of phenylphosphinic acid leads to highly ordered, covalently bound monolayers on TiO2 (110) without annealing. J. Phys. Chem. C. 121, 14213–14221 (2017).
-
Egerton, R. F. Radiation damage to organic and inorganic specimens in the TEM. Micron 119, 72–87 (2019).
-
Leijten, Z. J. W. A., Keizer, A. D. A., de With, G. & Friedrich, H. Quantitative analysis of electron beam damage in organic thin films. J. Phys. Chem. C 121, 10552–10561 (2017).
-
Lackey, H. E., Nelson, G. L., Lines, A. M. & Bryan, S. A. Reimagining pH measurement: utilizing Raman spectroscopy for enhanced accuracy in phosphoric acid systems. Anal. Chem. 92, 5882–5889 (2020).
-
Belding, L. et al. Conformation, and charge tunneling through molecules in SAMs. J. Am. Chem. Soc. 143, 3481–3493 (2021).
-
Bodo, E. et al. Interaction of a long alkyl chain protic ionic liquid and water. Chem. Phys. 140, 204503 (2014).
-
Lütgens, M., Chatzipapadopoulos, S. & Lochbrunner, S. Ultrafast CARS with improved spectral resolution. EPJ Web Conf. 41, 05007 (2013).
-
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
-
Shin, S.-J. et al. A unifying mechanism for cation effect modulating C1 and C2 productions from CO2 electroreduction. Nat. Commun. 13, 5482 (2022).
-
Wijesuriya, S., Burugapalli, K., Mackay, R., Ajaezi, G. C. & Balachandran, W. Chemically roughened solid silver: a simple, robust and broadband SERS substrate. Sensors 16, 1742 (2016).
-
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter 47, 558–561 (1993).
-
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
-
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 54, 11169–11186 (1996).
-
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter 59, 1758–1775 (1999).
-
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
-
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Chem. Phys. 132, 154104 (2010).
-
Wang, Y., Wang, W., Fan, K.-N. & Deng, J. Structural and electronic properties of silver surfaces: ab initio pseudopotential density functional study. Surf. Sci. 490, 125–132 (2001).
-
Shan, W., Liu, R., Zhao, H. & Liu, J. Bicarbonate rebalances the *COOH/*OCO– dual pathways in CO2 electrocatalytic reduction: in situ surface-enhanced Raman spectroscopic evidence. J. Phys. Chem. Lett. 13, 7296–7305 (2022).
-
Goldie, S. J., Bush, S., Cumming, J. A. & Coleman, K. S. A statistical approach to Raman analysis of graphene-related materials: implications for quality control. ACS Appl. Nano Mater. 3, 11229–11239 (2020).
Acknowledgements
This work was supported by the Director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, & Biosciences Division, of the US Department of Energy (DOE) under contract no. DE-AC02-05CH11231 and FWP CH030201 (Catalysis Research Program). This research used resources of the Advanced Light Source, a DOE Office of Science User Facility under the same contract number. Nano-FTIR was performed at beamlines 2.4 and 5.4 in ALS. XPS, SEM and STEM/energy-dispersive X-Ray were performed at the Molecular Foundry, supported by the Office of Science, Office of Basic Energy Sciences of DOE under contract number DE-AC02-05CH11231. Y.S. acknowledges fellowship from University of Chinese Academy of Sciences. X.Z. is supported by an NSF-BSF grant (no. 1906014). J.Q. is supported by the Laboratory Directed Research and Development Program of Lawrence Berkeley National Laboratory through contract no. DE-AC02-05CH11231, and by the Gas Phase Chemical Physics Program of the US DOE, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, through contract no. DE-AC02-05CH11231. A.J. is supported by an Early Career Award in the Condensed Phase and Interfacial Molecular Science Program, in the Chemical Sciences Geosciences and Biosciences Division of the Office of Basic Energy Sciences of the United States DoE under contract no. DE-AC02-05CH11231, and also by the Gas Phase Chemical Physics Program of the US DOE, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, through contract no. DE-AC02-05CH11231. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the US DOE, under contract no. DE-AC02-05CH11231, using NERSC award no. BES-ERCAP0020767. H.C. is supported by the Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, of the US DOE under contract nos. DE-AC02-05CH11231 and FWP CH030201 (Catalysis Research Program). We also thank J. Jin for the informative discussions.
Author information
Authors and Affiliations
Contributions
Y.S., X.Z. and M.F.G. designed this project under the guidance of M.B.S. and P.Y. Y.S. synthesized materials and conducted electrochemical and electron microscopy characterization, with the help of S.C., S.Y., I.R., H.C. and V.A. Y.S. and X.Z. performed the nano-FTIR experiment and analysed data, assisted by S.N.G.C. and H.A.B. M.F.G. conducted Raman measurements. A.J. and J.Q. performed DFT calculations. SFG measurements were conducted by K.C.N. and X.Z. All authors contributed to discussion of the experimental results and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks John Flake, Sangheon Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–7, methods, Figs. 1–21, Tables 1–7 and references.
Supplementary Data 1
Atomic coordinates of optimized bidentate structure.
Supplementary Data 2
Atomic coordinates of optimized monodentate structure.
Supplementary Data 3
Atomic coordinates of optimized free ligand structure.
Supplementary Video 1
The video shows the motion of vibrational mode with a wavenumber of 899 cm−1.
Supplementary Video 2
The video shows the motion of vibrational mode with a wavenumber of 969 cm−1.
Supplementary Video 3
The video shows the motion of vibrational mode with a wavenumber of 998 cm−1.
Supplementary Video 4
The video shows the motion of vibrational mode with a wavenumber of 1,006 cm−1.
Supplementary Video 5
The video shows the motion of vibrational mode with a wavenumber of 1,018 cm−1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shan, Y., Zhao, X., Fonseca Guzman, M. et al. Nanometre-resolved observation of electrochemical microenvironment formation at the nanoparticle–ligand interface. Nat Catal (2024). https://ift.tt/1bhZQWV
-
Received:
-
Accepted:
-
Published:
-
DOI: https://ift.tt/1bhZQWV
"interface" - Google News
March 25, 2024 at 05:58PM
https://ift.tt/WJ03m8c
Nanometre-resolved observation of electrochemical microenvironment formation at the nanoparticle–ligand interface - Nature.com
"interface" - Google News
https://ift.tt/g0BjxMO
https://ift.tt/sxVWhZQ
Bagikan Berita Ini

















0 Response to "Nanometre-resolved observation of electrochemical microenvironment formation at the nanoparticle–ligand interface - Nature.com"
Post a Comment