
The online cover of Science Advances this week features the assembly of nanoparticle surfactants at a solid-liquid interface using advanced microscopy techniques such as laser scanning confocal microscopy and atomic force microscopy. Materials scientists had explored the assembly of solids at a liquid interface for decades to understand ore (a complex and stable chemical compound) purification, emulsion and encapsulation processes. In a new report, Yu Chai and a research team at the Lawrence Berkeley National Laboratory, University of California Berkeley, the Hong Kong Polytechnic University and the Tohoku University, in the U.S., China and Japan, showed how electrostatic interactions between nanoparticles and ligands formed nanoparticle surfactants at water-oil interfaces. The resulting 'jammed' structures produced a solid-like layer. When the area density of the nanoparticle surfactants increased at the interface, further attachment required cooperative displacement of previously assembled nanoparticle surfactants. The high space-time resolution of their observations revealed the complex mechanism of attachment and the nature of nanoparticle assembly.
Observing solids at liquid interfaces
In this work, Chai et al. used atomic force microscopy (AFM) coupled with laser scanning confocal microscopy (LSCM) to obtain remarkable details of solids at liquid interfaces to provide insight into the nanoparticle jamming phenomena. Materials researchers in applied engineering are interested on the assembly of solids at liquid interfaces for applications such as ore purification, emulsion and encapsulation based on interfacial segregation. When the particle size decreases, the binding energy of the particle at the interface can decrease, resulting in the adsorption and desorption of nanoparticles. If nanoparticles that are soluble in one liquid interacts with end-functionalized ligands in a second immiscible liquid, researchers can increase the binding energy of nanoparticles to the interface to form nanoparticle surfactants. The very high binding energy of adsorption can drive the system to a non-equilibrium state.
Regulating the interfacial tension
The team characterized the interface between two immiscible liquids by calculating the interfacial tension (γ). When negatively charged nanoparticles were dispersed in the aqueous phase, the interfacial tension was not affected because the nanoparticles did not assemble at the interface due to the inherently negative charge at the water-oil interface. However, polymeric surfactants such as amine-terminated polydimethylsiloxane (PMDS-NH2), dissolved in silicone oil and assembled into a monolayer at the interface to reduce the interfacial tension. The magnitude of reduced interfacial tension depended on the concentration of PDMS-NH2 and molecular weight of the PDMS chain.

The team noted an attachment process, where carboxylic acid-functionalized nanoparticles diffused to the interface and interacted with cationic polymeric surfactants (PDMS-NH3+) to form nanoparticle surfactants. By labeling the nanoparticles with fluorescent markers, Chai et al. examined the adsorption process under low resolution using laser scanning confocal microscopy. The adsorption kinetics complied with Fick's Law; i.e., going from a high-concentration area to a low-concentration area proportional to the concentration gradient, with notable Fickian diffusion control of attachment. The results, therefore, supported diffusion-controlled adsorption to the interface, where the energy barrier to attachment was lower than the thermal energy of the system. The nanoparticles then remained at the interface after contacting the interface.
Using atomic force microscopy to distinguish nanoparticles
When more nanoparticles assembled at the water-oil interface, the laser scanning confocal microscopy technique could not effectively distinguish them individually—since the minimal separation distance exceeded the resolution of the instrument. The team therefore used atomic force microscopy, to directly visualize the attachment of nanoparticles on the water-oil interface in space-time. They then determined the diameters and position of nanoparticles relative to the interface and showed the binding energy of nanoparticles to the interface as a function of particle size and surface tension, at the oil-water interface. Based on the behavior of nanoparticles at the water-oil interface interface, Chai et al noted how the increasing charge density more strongly influenced the attachment of the surfactant to the nanoparticle, increasing its surface energy and driving the particles further into the oil phase. The movement dynamics of nanoparticles slowed down at the interface due to the more densely packed arrangement.
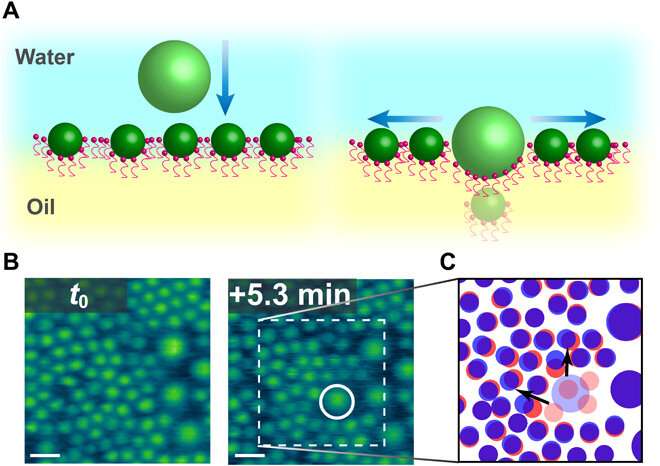
When the local area density of nanoparticles at the interface increased, there was insufficient space to accommodate the entry of new nanoparticles; therefore, the assembly rearranged on its own. Chai et al. noted this rearrangement using atomic force microscopy, although they did not quantify the observed fluctuations. They observed cooperative structural changes of assembled nanoparticles at the interface to accommodate the attachment of additional particles. Interestingly, several nanoparticles were not detectable, potentially trapped beneath larger nanoparticles added to the system; however, the team could not observe this phenomenon using atomic force microscopy alone. Chai et al. therefore reintegrated laser scanning confocal microscopy (LSCM) to the setup to provide insight to the addition of excess nanoparticles to the already dense assemblies.
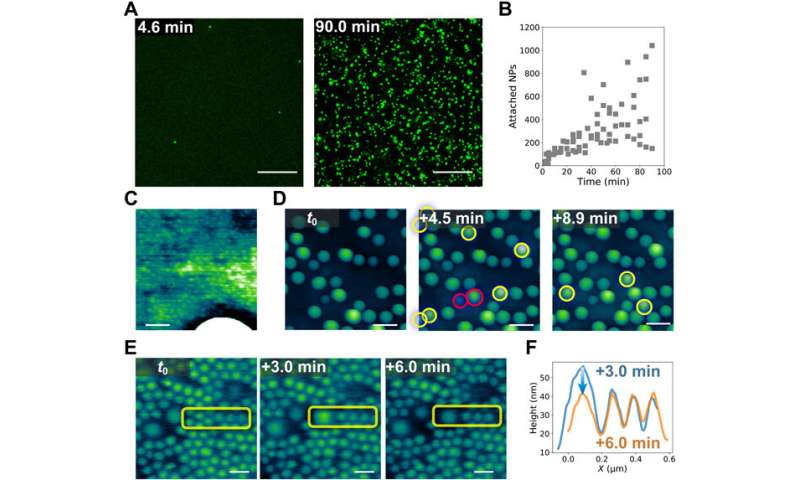
The scientists further incorporated LSCM (laser scanning confocal microscopy) experiments to investigate the mixed dispersion of nanoparticles of varying size to probe their dynamic co-assembly. While large and small particles co-assembled at the interface, only the large nanoparticles could be clearly resolved. Interestingly, the team noted many dark areas in the form of cracks, likely from the contact between water and oil phases in the setup. The crack formation further exposed new interfacial areas, which eventually self-healed as an important trademark of structured liquids to maintain their integrity in general.
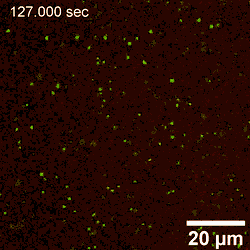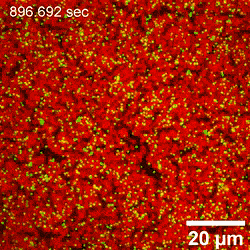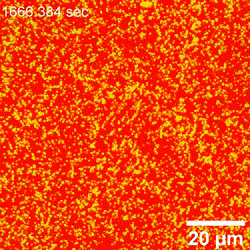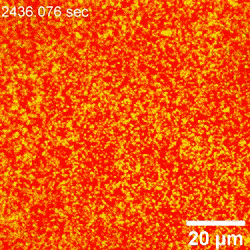
In this way, Yu Chai and colleagues investigated the assembly of nanoparticles at the water-oil interface and examined the factors controlling the process of adsorption. By interchangeably using AFM (atomic force microscopy) and LSCM (laser scanning confocal microscopy), they noted structural changes occurring at the early stage of nanoparticle attachment to the interface, including diffusion-controlled processes. The attachment process was reaction-controlled, where the existing assembly posed an electrostatic barrier to additional nanoparticles approaching the interface; thereby coordinating their rearrangement to accommodate the attachment of new nanoparticles. Using advanced microscopy techniques, the team detailed the attachment process under diverse conditions at high resolution to provide insight to adsorption and jamming in order to aid the design and fabrication of responsive assemblies.
Explore further
Crossley S. et al. Solid nanoparticles that catalyze biofuel upgrade reactions at the water/oil interface, Science, 10.1126/science.1180769
Kaz D. et al. Physical aging of the contact line on colloidal particles at liquid interfaces. Nature Materials, doi.org/10.1038/nmat3190
© 2020 Science X Network
Citation: Nanoparticle jamming at the water-oil interface (2020, December 4) retrieved 4 December 2020 from https://ift.tt/39H6YFD
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
"interface" - Google News
December 04, 2020 at 09:30PM
https://ift.tt/39H6YFD
Nanoparticle jamming at the water-oil interface - Phys.org
"interface" - Google News
https://ift.tt/2z6joXy
https://ift.tt/2KUD1V2
Bagikan Berita Ini














0 Response to "Nanoparticle jamming at the water-oil interface - Phys.org"
Post a Comment