Abstract
Invasive brain–machine interfaces can restore motor, sensory and cognitive functions. However, their clinical adoption has been hindered by the surgical risk of implantation and by suboptimal long-term reliability. In this Review, we highlight the opportunities and challenges of invasive technology for clinically relevant electrophysiology. Specifically, we discuss the characteristics of neural probes that are most likely to facilitate the clinical translation of invasive neural interfaces, describe the neural signals that can be acquired or produced by intracranial electrodes, the abiotic and biotic factors that contribute to their failure, and emerging neural-interface architectures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
79,00 € per year
only 6,58 € per issue
Rent or buy this article
Get just this article for as long as you need it
$39.95
Prices may be subject to local taxes which are calculated during checkout
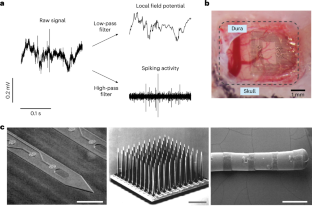

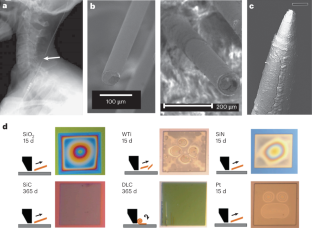
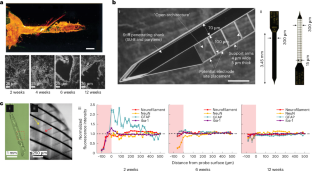
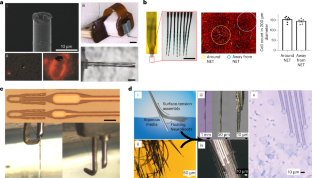
References
-
Lebedev, M. A. & Nicolelis, M. A. L. Brain–machine interfaces: from basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 97, 767–837 (2017).
-
Min, B.-K., Marzelli, M. J. & Yoo, S.-S. Neuroimaging-based approaches in the brain–computer interface. Trends Biotechnol. 28, 552–560 (2010).
-
Nicolas-Alonso, L. F. & Gomez-Gil, J. Brain computer interfaces, a review. Sensors 12, 1211–1279 (2012).
-
Naseer, N. & Hong, K.-S. fNIRS-based brain-computer interfaces: a review. Front. Hum. Neurosci. 9, 3 (2015).
-
Hong, K.-S., Ghafoor, U. & Khan, M. J. Brain–machine interfaces using functional near-infrared spectroscopy: a review. Artif. Life Robot. 25, 204–218 (2020).
-
Thibault, R. T., MacPherson, A., Lifshitz, M., Roth, R. R. & Raz, A. Neurofeedback with fMRI: a critical systematic review. Neuroimage 172, 786–807 (2018).
-
Tonin, L. & Millán, Jd. R. Noninvasive brain–machine interfaces for robotic devices. Annu. Rev. Control Robot. Auton. Syst. 4, 191–214 (2020).
-
Bullard, J., Hutchison, B. C., Lee, J., Chestek, C. A. & Patil, P. G. Estimating risk for future intracranial, fully implanted, modular neuroprosthetic systems: a systematic review of hardware complications in clinical deep brain stimulation and experimental human intracortical arrays. Neuromodulation 23, 411–426 (2019).
-
Anderson, D. N., Osting, B., Vorwerk, J., Dorval, A. D. & Butson, C. R. Optimized programming algorithm for cylindrical and directional deep brain stimulation electrodes. J. Neural Eng. 15, 026005 (2018).
-
Skarpaas, T. L., Jarosiewicz, B. & Morrell, M. J. Brain-responsive neurostimulation for epilepsy (RNS® System). Epilepsy Res. 153, 68–70 (2019).
-
Stieglitz, T. Of man and mice: translational research in neurotechnology. Neuron 105, 12–15 (2020).
-
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
-
Won, S. M., Song, E., Reeder, J. T. & Rogers, J. A. Emerging modalities and implantable technologies for neuromodulation. Cell 181, 115–135 (2020).
-
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
-
Frank, J. A., Antonini, M.-J. & Anikeeva, P. Next-generation interfaces for studying neural function. Nat. Biotechnol. 37, 1013–1023 (2019).
-
Wellman, S. M. et al. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 28, 1701269 (2018).
-
Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345 (2019).
-
Pei, F. & Tian, B. Nanoelectronics for minimally invasive cellular recordings. Adv. Funct. Mater. 30, 1906210 (2019).
-
Abbott, J., Ye, T., Ham, D. & Park, H. Optimizing nanoelectrode arrays for scalable intracellular electrophysiology. Acc. Chem. Res. 51, 600–608 (2018).
-
Annecchino, L. A. & Schultz, S. R. Progress in automating patch clamp cellular physiology. Brain Neurosci. Adv. 2, 2398212818776561 (2018).
-
Zhang, A., Zhao, Y., You, S. S. & Lieber, C. M. Nanowire probes could drive high-resolution brain-machine interfaces. Nano Today 31, 100821 (2020).
-
Jouhanneau, J.-S., Kremkow, J., Dorrn, A. L. & Poulet, J. F. A. In vivo monosynaptic excitatory transmission between layer 2 cortical pyramidal neurons. Cell Rep. 13, 2098–2106 (2015).
-
Kodandaramaiah, S. B., Franzesi, G. T., Chow, B. Y., Boyden, E. S. & Forest, C. R. Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat. Methods 9, 585–587 (2012).
-
Kodandaramaiah, S. B. et al. Multi-neuron intracellular recording in vivo via interacting autopatching robots. eLife 7, e24656 (2018).
-
Holst, G. L. et al. Autonomous patch-clamp robot for functional characterization of neurons in vivo: development and application to mouse visual cortex. J. Neurophysiol. 121, 2341–2357 (2019).
-
Dubey, A. & Ray, S. Cortical electrocorticogram (ECoG) is a local signal. J. Neurosci. 39, 4299–4311 (2019).
-
Yanagisawa, T. et al. Real-time control of a prosthetic hand using human electrocorticography signals. J. Neurosurg. 114, 1715–1722 (2011).
-
Anumanchipalli, G. K., Chartier, J. & Chang, E. F. Speech synthesis from neural decoding of spoken sentences. Nature 568, 493–498 (2019).
-
Nune, G. et al. Treatment of drug-resistant epilepsy in patients with periventricular nodular heterotopia using RNS® System: efficacy and description of chronic electrophysiological recordings. Clin. Neurophysiol. 130, 1196–1207 (2019).
-
Vansteensel, M. J. et al. Fully implanted brain–computer interface in a locked-in patient with ALS. N. Engl. J. Med. 375, 2060–2066 (2016).
-
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
-
Khodagholy, D. et al. Organic electronics for high-resolution electrocorticography of the human brain. Sci. Adv. 2, e1601027 (2016).
-
Ledochowitsch, P. et al. Fabrication and testing of a large area, high density, parylene MEMS μECoG array. In IEEE 24th International Conference on Micro Electro Mechanical Systems 1031–1034 (IEEE, 2011).
-
Muller, L. et al. Thin-film, high-density micro-electrocorticographic decoding of a human cortical gyrus. In 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 1528–1531 (IEEE, 2016).
-
Jackson, A. & Fetz, E. E. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. J. Neurophysiol. 98, 3109–3118 (2007).
-
Williams, J. C., Rennaker, R. L. & Kipke, D. R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 4, 303–313 (1999).
-
Ferguson, J. E., Boldt, C. & Redish, A. D. Creating low-impedance tetrodes by electroplating with additives. Sens. Actuators A 156, 388–393 (2009).
-
Schwarz, D. A. et al. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat. Methods 11, 670–676 (2014).
-
Kollo, M. et al. CHIME: CMOS-hosted in-vivo microelectrodes for massively scalable neuronal recordings. Front. Neurosci. https://doi.org/10.3389/fnins.2020.00834 (2020).
-
Obaid, A. et al. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 6, eaay2789 (2020).
-
Kipke, D. R., Vetter, R. J., Williams, J. C. & Hetke, J. F. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 11, 151–155 (2003).
-
Jones, K. E., Campbell, K. & Normann, R. A. A glass/silicon composite intracortical electrode array. Ann. Biomed. Eng. 20, 423–437 (1992).
-
Li, Z. Decoding methods for neural prostheses: where have we reached? Front. Syst. Neurosci. 8, 129 (2014).
-
Kao, J. C., Stavisky, S. D., Sussillo, D., Nuyujukian & Shenoy, K. V. Information systems opportunities in brain–machine interface decoders. Proc. IEEE 102, 666–682 (2014).
-
Shenoy, K. V. & Carmena, J. M. Combining decoder design and neural adaptation in brain–machine interfaces. Neuron 84, 665–680 (2014).
-
Pandarinath, C. et al. Inferring single-trial neural population dynamics using sequential auto-encoders. Nat. Methods 15, 805–815 (2018).
-
Turner, J. N. et al. Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 156, 33–49 (1999).
-
Biran, R., Martin, D. C. & Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195, 115–126 (2005).
-
Lebedev, M. A., Crist, R. E. & Nicolelis, M. A. L. in Methods for Neural Ensemble Recordings 2nd edn (ed Nicolelis, M. A. L.) Ch. 11 (CRC Press/Taylor & Francis, 2007).
-
Miller, E. K., Lundqvist, M. & Bastos, A. M. Working Memory 2.0. Neuron 100, 463–475 (2018).
-
Collinger, J. L. et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381, 557–564 (2013).
-
Ajiboye, A. B. et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet 389, 1821–1830 (2017).
-
Nuyujukian, P. et al. Cortical control of a tablet computer by people with paralysis. PLoS ONE 13, e0204566 (2018).
-
Degenhart, A. D. et al. Stabilization of a brain–computer interface via the alignment of low-dimensional spaces of neural activity. Nat. Biomed. Eng. 4, 672–685 (2020).
-
Merrill, D. R., Bikson, M. & Jefferys, J. G. R. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods 141, 171–198 (2005).
-
Robinson, J. T. et al. Developing next-generation brain sensing technologies–a review. IEEE Sens. J. 19, 10163–10175 (2019).
-
Cogan, S. F. Microelectrode coatings for neural stimulation and recording. In Proc. 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 3798–3801 (IEEE, 2003).
-
Chouard, C. H. & Pialoux, P. Biocompatibility of cochlear implants. Bull. Acad. Natl Med. 179, 549–555 (1995).
-
Majji, A. B. et al. Long-term histological and electrophysiological results of an inactive epiretinal electrode array implantation in dogs. Invest. Ophthalmol. Vis. Sci. 40, 2073–2081 (1999).
-
Stronks, H. C. & Dagnelie, G. The functional performance of the Argus II retinal prosthesis. Expert Rev. Med. Devices 11, 23–30 (2014).
-
Sun, F. T. & Morrell, M. J. The RNS System: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev. Med. Devices 11, 563–572 (2014).
-
Musk, E. et al. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
-
Keefer, E. W., Botterman, B. R., Romero, M. I., Rossi, A. F. & Gross, G. W. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 3, 434–439 (2008).
-
Venkatraman, S. et al. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 307–316 (2011).
-
Bédard, C., Kröger, H. & Destexhe, A. Modeling extracellular field potentials and the frequency-filtering properties of extracellular space. Biophys. J. 86, 1829–1842 (2004).
-
Marblestone, A. et al. Physical principles for scalable neural recording. Front. Comput. Neurosci. 7, 137 (2013).
-
Kleinfeld, D. et al. Can one concurrently record electrical spikes from every neuron in a mammalian brain? Neuron 103, 1005–1015 (2019).
-
Robinson, D. A. The electrical properties of metal microelectrodes. Proc. IEEE 56, 1065–1071 (1968).
-
Nyquist, H. Thermal agitation of electric charge in conductors. Phys. Rev. 32, 110 (1928).
-
Hassibi, A., Navid, R., Dutton, R. W. & Lee, T. H. Comprehensive study of noise processes in electrode electrolyte interfaces. J. Appl. Phys. 96, 1074–1082 (2004).
-
Grill, W. M. Safety considerations for deep brain stimulation: review and analysis. Expert Rev. Med. Devices 2, 409–420 (2005).
-
Hudak, E. M., Mortimer, J. T. & Martin, H. B. Platinum for neural stimulation: voltammetry considerations. J. Neural Eng. 7, 026005 (2010).
-
Cogan, S. F., Ludwig, K. A., Welle, C. G. & Takmakov, P. Tissue damage thresholds during therapeutic electrical stimulation. J. Neural Eng. 13, 021001 (2016).
-
Cogan, S. F., Hara, S. & Ludwig, K. A. in Neuromodulation (eds Krames, E. S. et al.) 83–94 (Elsevier, 2018).
-
Huang, C. Q., Carter, M. & Shepherd, R. K. Stimulus induced pH changes in cochlear implants: an in vitro and in vivo study. Ann. Biomed. Eng. 29, 791–802 (2001).
-
Crago, P. E., Peckham, H., Mortimer, J. T. & Van Der Meulen, J. P. The choice of pulse duration for chronic electrical stimulation via surface, nerve, and intramuscular electrodes. Ann. Biomed. Eng. 2, 252–264 (1974).
-
Grill, W. M. & Mortimer, J. T. The effect of stimulus pulse duration on selectivity of neural stimulation. IEEE Trans. Biomed. Eng. 43, 161–166 (1996).
-
Robblee, L. S., McHardy, J., Agnew, W. F. & Bullara, L. A. Electrical stimulation with Pt electrodes. VII. Dissolution of Pt electrodes during electrical stimulation of the cat cerebral cortex. J. Neurosci. Methods 9, 301–308 (1983).
-
Brummer, S. B. & Turner, M. J. Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans. Biomed. Eng. BME-24, 59–63 (1977).
-
McHardy, J., Robblee, L. S., Marston, J. M. & Brummer, S. B. Electrical stimulation with Pt electrodes. IV. Factors influencing Pt dissolution in inorganic saline. Biomaterials 1, 129–134 (1980).
-
Guyton, D. L. & Hambrecht, F. T. Theory and design of capacitor electrodes for chronic stimulation. Med. Biol. Eng. 12, 613–620 (1974).
-
Agnew, W. F., Yuen, T. G. H., McCreery, D. B. & Bullara, L. A. Histopathologic evaluation of prolonged intracortical electrical stimulation. Exp. Neurol. 92, 162–185 (1986).
-
Lempka, S. F., Johnson, M. D., Miocinovic, S., Vitek, J. L. & McIntyre, C. C. Current-controlled deep brain stimulation reduces in vivo voltage fluctuations observed during voltage-controlled stimulation. Clin. Neurophysiol. 121, 2128–2133 (2010).
-
Zeng, F.-G., Rebscher, S., Harrison, W., Sun, X. & Feng, H. Cochlear implants: system design, integration, and evaluation. IEEE Rev. Biomed. Eng. 1, 115–142 (2008).
-
Salas, M. A. et al. Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. eLife 7, e32904 (2018).
-
Zhou, D. D., Dorn, J. D. & Greenberg, R. J. The Argus® II retinal prosthesis system: an overview’. In IEEE International Conference on Multimedia and Expo Workshops (ICMEW) 1–6 (IEEE, 2013).
-
Hambrecht, F. T. Visual prostheses based on direct interfaces with the visual system. Baillieres Clin. Neurol. 4, 147–165 (1995).
-
Leung, R. T., Shivdasani, M. N., Nayagam, D. A. X. & Shepherd, R. K. In vivo and in vitro comparison of the charge injection capacity of platinum macroelectrodes. IEEE Trans. Biomed. Eng. 62, 849–857 (2014).
-
Kane, S. R. et al. Electrical performance of penetrating microelectrodes chronically implanted in cat cortex. IEEE Trans. Biomed. Eng. 60, 2153–2160 (2013).
-
Ludwig, K. A. et al. Poly (3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes. J. Neural Eng. 8, 014001 (2011).
-
Cui, X. & Martin, D. C. Electrochemical deposition and characterization of poly (3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens. Actuators B 89, 92–102 (2003).
-
Thaning, E. M., Asplund, M. L. M., Nyberg, T. A., Inganäs, O. W. & Holst, H. Stability of poly (3,4-ethylene dioxythiophene) materials intended for implants. J. Biomed. Mater. Res. B 93, 407–415 (2010).
-
Leber, M. et al. Long term performance of porous platinum coated neural electrodes. Biomed. Microdevices 19, 62 (2017).
-
Aryan, N. P. et al. In vitro study of titanium nitride electrodes for neural stimulation. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2866–2869 (IEEE, 2011).
-
Cogan, S. F., Guzelian, A. A., Agnew, W. F., Yuen, T. G. H. & McCreery, D. B. Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation. J. Neurosci. Methods 137, 141–150 (2004).
-
Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
-
Jorfi, M., Skousen, J. L., Weder, C. & Capadona, J. R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 12, 011001 (2014).
-
Kuliasha, C. A. & Judy, J. W. In vitro reactive-accelerated-aging assessment of anisotropic conductive adhesive and back-end packaging for electronic neural interfaces. In 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 3766–3769 (IEEE, 2019).
-
Patrick, E., Orazem, M. E., Sanchez, J. C. & Nishida, T. Corrosion of tungsten microelectrodes used in neural recording applications. J. Neurosci. Methods 198, 158–171 (2011).
-
Bowman, L. & Meindl, J. D. The packaging of implantable integrated sensors. IEEE Trans. Biomed. Eng. 33, 248–255 (1986).
-
Barrese, J. C., Aceros, J. & Donoghue, J. P. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates. J. Neural Eng. 13, 026003 (2016).
-
Prasad, A. et al. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 9, 056015 (2012).
-
Prasad, A. et al. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 7, 2 (2014).
-
Ordonez, J. S., Boehler, C., Schuettler, M. & Stieglitz, T. Improved polyimide thin-film electrodes for neural implants. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society 5134–5137 (IEEE, 2012).
-
Ordonez, J. S., Boehler, C., Schuettler, M. & Stieglitz, T. Long-term adhesion studies of polyimide to inorganic and metallic layers. MRS Online Proc. Library https://doi.org/10.1557/opl.2012.1198 (2012).
-
Ordonez, J. S., Boehler, C., Schuettler, M. & Stieglitz, T. Silicone rubber and thin-film polyimide for hybrid neural interfaces—a MEMS-based adhesion promotion technique. In 6th International IEEE/EMBS Conference on Neural Engineering (NER) 872–875 (IEEE, 2013).
-
Kuliasha, C. A. & Judy, J. W. In vitro reactive-accelerated-aging (RAA) assessment of tissue-engineered electronic nerve interfaces (TEENI). In 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 5061–5064 (IEEE, 2018).
-
Loeb, G. E., Bak, M. J., Salcman, M. & Schmidt, E. M. Parylene as a chronically stable, reproducible microelectrode insulator. IEEE Trans. Biomed. Eng. 24, 121–128 (1977).
-
Traeger, R. Nonhermeticity of polymeric lid sealants. IEEE Trans. Parts Hybrids Packaging 13, 147–152 (1977).
-
Vanhoestenberghe, A. & Donaldson, N. Corrosion of silicon integrated circuits and lifetime predictions in implantable electronic devices. J. Neural Eng. 10, 031002 (2013).
-
Hassler, C., Metzen, R. P., Ruther & Stieglitz, T. Characterization of parylene C as an encapsulation material for implanted neural prostheses. J. Biomed. Mater. Res. B 93, 266–274 (2010).
-
Von Metzen, R. P. & Stieglitz, T. The effects of annealing on mechanical, chemical, and physical properties and structural stability of parylene C. Biomed. Microdevices 15, 727–735 (2013).
-
Kim, B. J., Washabaugh, E. P. & Meng, E. Annealing effects on flexible multi-layered parylene-based sensors. In IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS) 825–828 (IEEE, 2014).
-
Gwon, T. M., Kim, J. H., Choi, G. J. & Kim, S. J. Mechanical interlocking to improve metal–polymer adhesion in polymer-based neural electrodes and its impact on device reliability. J. Mater. Sci. 51, 6897–6912 (2016).
-
Kim, W.-S., Yun, I.-H., Lee, J.-J. & Jung, H.-T. Evaluation of mechanical interlock effect on adhesion strength of polymer–metal interfaces using micro-patterned surface topography. Int. J. Adhes. Adhes. 30, 408–417 (2010).
-
Fang, H. et al. Ultrathin, transferred layers of thermally grown silicon dioxide as biofluid barriers for biointegrated flexible electronic systems. Proc. Natl Acad. Sci. USA 113, 11682–11687 (2016).
-
Xie, X. et al. Long-term reliability of Al2O3 and parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J. Neural Eng. 11, 026016 (2014).
-
Maloney, J. M., Lipka, S. A. & Baldwin, S. P. In vivo biostability of CVD silicon oxide and silicon nitride films. MRS Online Proc. Library 872, 143 (2005).
-
Jeong, J. et al. Conformal hermetic sealing of wireless microelectronic implantable chiplets by multilayered atomic layer deposition (ALD). Adv. Funct. Mater. 29, 1806440 (2019).
-
Cogan, S. F., Edell, D. J., Guzelian, A. A., Ping Liu, Y. & Edell, R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J. Biomed. Mater. Res. A 67, 856–867 (2003).
-
Hsu, J.-M., Tathireddy, Rieth, L., Normann, A. R. & Solzbacher, F. Characterization of a-SiCx:H thin films as an encapsulation material for integrated silicon based neural interface devices. Thin Solid Films 516, 34–41 (2007).
-
Lei, X. et al. SiC protective coating for photovoltaic retinal prosthesis. J. Neural Eng. 13, 046016 (2016).
-
Knaack, G. L. et al. In vivo characterization of amorphous silicon carbide as a biomaterial for chronic neural interfaces. Front. Neurosci. 10, 301 (2016).
-
Phan, H.-P. et al. Long-lived, transferred crystalline silicon carbide nanomembranes for implantable flexible electronics. ACS Nano 13, 11572–11581 (2019).
-
Diaz-Botia, C. A. et al. A silicon carbide array for electrocorticography and peripheral nerve recording. J. Neural Eng. 14, 056006 (2017).
-
Beygi, M. et al. Fabrication of a monolithic implantable neural interface from cubic silicon carbide. Micromachines 10, 430 (2019).
-
Polikov, V. S., Tresco, A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
-
Saxena, T. et al. The impact of chronic blood–brain barrier breach on intracortical electrode function. Biomaterials 34, 4703–4713 (2013).
-
Kozai, T. D. Y., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C. & Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 6, 48–67 (2015).
-
Kozai, T. D. Y., Vazquez, A. L., Weaver, C. L., Kim, S.-G. & Cui, X. T. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J. Neural Eng. 9, 066001 (2012).
-
Seymour, J. P. & Kipke, D. R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 28, 3594–3607 (2007).
-
Skousen, J. L. et al. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog. Brain Res. 194, 167–180 (2011).
-
Sanders, J. E., Stiles, C. E. & Hayes, C. L. Tissue response to single-polymer fibers of varying diameters: evaluation of fibrous encapsulation and macrophage density. J. Biomed. Mater. Res. 52, 231–237 (2000).
-
Yang, Q. et al. Zwitterionic polymer coating suppresses microglial encapsulation to neural implants in vitro and in vivo. Adv. Biosyst. 4, 1900287 (2020).
-
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
-
Fu, T.-M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016).
-
Zhou, T. et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl Acad. Sci. USA 114, 5894–5899 (2017).
-
Biran, R., Martin, D. C. & Tresco, P. A. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. A 82, 169–178 (2007).
-
Shen, K. & Maharbiz, M. M. Ceramic packaging in neural implants. J. Neural Eng. 18, 025002 (2021).
-
Hochberg, L. R. et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006).
-
Kim, S.-P., Simeral, J. D., Hochberg, L. R., Donoghue, J. P. & Black, M. J. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J. Neural Eng. 5, 455–476 (2008).
-
Kim, S.-P. et al. Point-and-click cursor control with an intracortical neural interface system by humans with tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 193–203 (2011).
-
Simeral, J. D., Kim, S.-P., Black, M. J., Donoghue, J. P. & Hochberg, L. R. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J. Neural Eng. 8, 025027 (2011).
-
Hochberg, L. R. et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012).
-
Kim, S. et al. Integrated wireless neural interface based on the Utah electrode array. Biomed. Microdevices 11, 453–466 (2009).
-
Rios, G., Lubenov, E. V., Chi, D., Roukes, M. L. & Siapas, A. G. Nanofabricated neural probes for dense 3-D recordings of brain activity. Nano Lett. 16, 6857–6862 (2016).
-
Seymour, J. P., Wu, F., Wise, K. D. & Yoon, E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 3, 16066 (2017).
-
Massey, T. L. et al. A high-density carbon fiber neural recording array technology. J. Neural Eng. 16, 016024 (2019).
-
Gillis, W. F. et al. Carbon fiber on polyimide ultra-microelectrodes. J. Neural Eng. 15, 016010 (2018).
-
Guitchounts, G., Markowitz, J. E., Liberti, W. A. & Gardner, T. J. A carbon-fiber electrode array for long-term neural recording. J. Neural Eng. 10, 046016 (2013).
-
Patel, P. R. et al. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 12, 046009 (2015).
-
Patel, P. R. et al. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J. Neural Eng. 13, 066002 (2016).
-
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 3, e1601966 (2017).
-
Wei, X. et al. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording. Adv. Sci. 5, 1700625 (2018).
-
Hanson, T. L., Diaz-Botia, C. A., Kharazia, V., Maharbiz, M. M. & Sabes, P. N. The “sewing machine” for minimally invasive neural recording. Preprint at bioRxiv https://doi.org/10.1101/578542 (2019).
-
Du, Z. J. et al. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 53, 46–58 (2017).
-
Ferro, M. D. et al. NeuroRoots, a bio-inspired, seamless Brain Machine Interface device for long-term recording. Preprint at bioRxiv https://doi.org/10.1101/460949 (2018).
-
Na, K. et al. Novel diamond shuttle to deliver flexible neural probe with reduced tissue compression. Microsyst. Nanoeng. 6, 37 (2020).
-
Chen, P.-C. & Lal, A. Detachable ultrasonic enabled inserter for neural probe insertion using biodissolvable polyethylene glycol. In 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 125–128 (IEEE, 2015).
-
Barz, F., Ruther, Takeuchi, S. & Paul, O. Flexible silicon-polymer neural probe rigidified by dissolvable insertion vehicle for high-resolution neural recording with improved duration. In 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS) 636–639 (IEEE, 2015).
-
Ceyssens, F. et al. Chronic neural recording with probes of subcellular cross-section using 0.06 mm² dissolving microneedles as insertion device. Sens. Actuators B 284, 369–376 (2019).
-
Kim, D.-H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010).
-
Piore, A. To study the brain, a doctor puts himself under the knife. MIT Technology Review https://www.technologyreview.com/2015/11/09/247535/to-study-the-brain-a-doctor-puts-himself-under-the-knife/ (2015).
-
Kennedy, P. R. The cone electrode: a long-term electrode that records from neurites grown onto its recording surface. J. Neurosci. Methods 29, 181–193 (1989).
-
Bartels, J. et al. Neurotrophic electrode: method of assembly and implantation into human motor speech cortex. J. Neurosci. Methods 174, 168–176 (2008).
-
Gearing, M. & Kennedy, P. Histological confirmation of myelinated neural filaments within the tip of the neurotrophic electrode after a decade of neural recordings. Front. Hum. Neurosci. 14, 111 (2020).
-
Xie, C. et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 14, 1286–1292 (2015).
-
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
-
Olsson, R. H. & Wise, K. D. A three-dimensional neural recording microsystem with implantable data compression circuitry. IEEE J. Solid State Circuits 40, 2796–2804 (2005).
-
Ruther, P. & Paul, O. New approaches for CMOS-based devices for large-scale neural recording. Curr. Opin. Neurobiol. 32, 31–37 (2015).
-
Ng, K. A., Greenwald, E., Xu, Y. P. & Thakor, N. V. Implantable neurotechnologies: a review of integrated circuit neural amplifiers. Med. Biol. Eng. Comput. 54, 45–62 (2016).
-
Wang, P.-M. et al. in Interfacing Bioelectronics and Biomedical Sensing (eds Cao, H. et al.) 1–28 (Springer, 2020).
-
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
-
Raducanu, B. C. et al. Time multiplexed active neural probe with 1356 parallel recording sites. Sensors 17, 2388 (2017).
-
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31 (2019).
-
Viventi, J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14, 1599–1605 (2011).
-
Patolsky, F. et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313, 1100–1104 (2006).
-
Steinmetz, N. A., Koch, C., Harris, K. D. & Carandini, M. Challenges and opportunities for large-scale electrophysiology with Neuropixels probes. Curr. Opin. Neurobiol. 50, 92–100 (2018).
-
Borton, D. A., Yin, M., Aceros, J. & Nurmikko, A. An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 10, 026010 (2013).
-
Muller, R. et al. A minimally invasive 64-channel wireless μECoG implant. IEEE J. Solid State Circuits 50, 344–359 (2014).
-
Thelin, J. et al. Implant size and fixation mode strongly influence tissue reactions in the CNS. PLoS ONE 6, e16267 (2011).
-
Leung, V. W. et al. A CMOS distributed sensor system for high-density wireless neural implants for brain-machine interfaces. In IEEE 44th European Solid State Circuits Conference (ESSCIRC) 230–233 (IEEE, 2018).
-
Seo, D., Carmena, J. M., Rabaey, J. M., Alon, E. & Maharbiz, M. M. Neural dust: an ultrasonic, low power solution for chronic brain–machine interfaces. Preprint at https://arxiv.org/abs/1307.2196 (2013).
-
Lee, J. et al. An implantable wireless network of distributed microscale sensors for neural applications. In 9th International IEEE/EMBS Conference on Neural Engineering (NER) 871–874 (IEEE, 2019).
-
Neely, R. M., Piech, D. K., Santacruz, S. R., Maharbiz, M. M. & Carmena, J. M. Recent advances in neural dust: towards a neural interface platform. Curr. Opin. Neurobiol. 50, 64–71 (2018).
-
Seo, D., Carmena, J. M., Rabaey, J. M., Maharbiz, M. M. & Alon, E. Model validation of untethered, ultrasonic neural dust motes for cortical recording. J. Neurosci. Methods 244, 114–122 (2015).
-
Piech, D. K. et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 4, 207–222 (2020).
-
Seo, D. et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 91, 529–539 (2016).
-
Ghanbari, M. M. et al. 17.5 A 0.8mm3 ultrasonic implantable wireless neural recording system with linear AM backscattering. In IEEE International Solid-State Circuits Conference (ISSCC) 284–286 (IEEE, 2019).
-
Charthad, J., Weber, M. J., Chang, T. C. & Arbabian, A. A mm-sized implantable medical device (IMD) with ultrasonic power transfer and a hybrid bi-directional data link. IEEE J. Solid State Circuits 50, 1741–1753 (2015).
-
Charthad, J. et al. A mm-sized wireless implantable device for electrical stimulation of peripheral nerves. IEEE Trans. Biomed. Circuits Syst. 12, 257–270 (2018).
-
Shi, C., Costa, T., Elloian, J., Zhang, Y. & Shepard, K. A 0.065-mm3 monolithically-integrated ultrasonic wireless sensing mote for real-time physiological temperature monitoring. IEEE Trans. Biomed. Circuits Syst. 14, 412–424 (2020).
-
Phillips, W. B., Towe, B. C. & Larson, P. J. An ultrasonically-driven piezoelectric neural stimulator. In Proc. 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 1983–1986 (IEEE, 2003).
-
Weber, M. J. et al. A miniaturized single-transducer implantable pressure sensor with time-multiplexed ultrasonic data and power links. IEEE J. Solid State Circuits 53, 1089–1101 (2018).
-
Larson, P. J. & Towe, B. C. Miniature ultrasonically powered wireless nerve cuff stimulator. In 5th International IEEE/EMBS Conference on Neural Engineering 265–268 (IEEE, 2011).
-
Sonmezoglu, S. & Maharbiz, M. M. 34.4 A 4.5mm3 deep-tissue ultrasonic implantable luminescence oxygen sensor. In IEEE International Solid-State Circuits Conference (ISSCC) 454–456 (IEEE, 2020).
-
Cortese, A. J. et al. Microscopic sensors using optical wireless integrated circuits. Proc. Natl Acad. Sci. USA 117, 9173–9179 (2020).
-
Stocking, K. C., Vazquez, A. L. & Kozai, T. D. Y. Intracortical neural stimulation with untethered, ultrasmall carbon fiber electrodes mediated by the photoelectric effect. IEEE Trans. Biomed. Eng. 66, 2402–2412 (2019).
-
Abdo, A. et al. Floating light-activated microelectrical stimulators tested in the rat spinal cord. J. Neural Eng. 8, 056012 (2011).
-
Singer, A. et al. Magnetoelectric materials for miniature, wireless neural stimulation at therapeutic frequencies. Neuron 107, 631–643 (2020).
-
Deer, T. R. et al. The Neurostimulation Appropriateness Consensus Committee (NACC) safety guidelines for the reduction of severe neurological injury. Neuromodulation 20, 15–30 (2017).
-
Rousche, P. J. & Normann, R. A. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann. Biomed. Eng. 20, 413–422 (1992).
-
Couldwell, W. T. et al. Computer-aided design/computer-aided manufacturing skull base drill. Neurosurg. Focus 42, E6 (2017).
-
Sato, T., Suzuki, T. & Mabuchi, K. A new multi-electrode array design for chronic neural recording, with independent and automatic hydraulic positioning. J. Neurosci. Methods 160, 45–51 (2007).
-
Jackson, N. et al. Long-term neural recordings using MEMS based moveable microelectrodes in the brain. Front. Neuroeng. 3, 10 (2010).
-
Fee, M. S. & Leonardo, A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J. Neurosci. Methods 112, 83–94 (2001).
-
Muthuswamy, J., Anand, S. & Sridharan, A. Adaptive movable neural interfaces for monitoring single neurons in the brain. Front. Neurosci. 5, 94 (2011).
-
Zoll, R. S. et al. MEMS-actuated carbon fiber microelectrode for neural recording. IEEE Trans. Nanobiosci. 18, 234–239 (2019).
-
Stieglitz, T. Why neurotechnologies? About the purposes, opportunities and limitations of neurotechnologies in clinical applications. Neuroethics 14, 5–16 (2019).
-
Eaton, M. L. & Illes, J. Commercializing cognitive neurotechnology—the ethical terrain. Nat. Biotechnol. 25, 393–397 (2007).
-
Thakor, N. V. Translating the brain-machine interface. Sci. Transl. Med. 5, 210ps17 (2013).
-
Koch, J., Schuettler, M., Pasluosta, C. & Stieglitz, T. Electrical connectors for neural implants: design, state of the art and future challenges of an underestimated component. J. Neural Eng. 16, 061002 (2019).
-
Rose, T. L. & Robblee, L. S. Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses (neuronal application). IEEE Trans. Biomed. Eng. 37, 1118–1120 (1990).
-
Negi, S., Bhandari, R., Rieth, L. & Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 5, 015007 (2010).
-
Weremfo, A., Carter, Hibbert, D. B. & Zhao, C. Investigating the interfacial properties of electrochemically roughened platinum electrodes for neural stimulation. Langmuir 31, 2593–2599 (2015).
-
Boehler, C., Oberueber, F., Schlabach, S., Stieglitz, T. & Asplund, M. Long-term stable adhesion for conducting polymers in biomedical applications: IrOx and nanostructured platinum solve the chronic challenge. ACS Appl. Mater. Interfaces 9, 189–197 (2017).
-
Weiland, J. D., Anderson, D. J. & Humayun, M. S. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans. Biomed. Eng. 49, 1574–1579 (2002).
-
Cogan, S. F., Troyk, R., Ehrlich, J., Plante, T. D. & Detlefsen, D. E. Potential-biased, asymmetric waveforms for charge-injection with activated iridium oxide (AIROF) neural stimulation electrodes. IEEE Trans. Biomed. Eng. 53, 327–332 (2006).
-
Ghazavi, A., Maeng, J., Black, M., Salvi, S. & Cogan, S. F. Electrochemical characteristics of ultramicro-dimensioned SIROF electrodes for neural stimulation and recording. J. Neural Eng. 17, 016022 (2020).
-
Deku, F., Joshi-Imre, A., Mertiri, A., Gardner, T. J. & Cogan, S. F. Electrodeposited iridium oxide on carbon fiber ultramicroelectrodes for neural recording and stimulation. J. Electrochem. Soc. 165, D375 (2018).
-
Zhou, D. M. & Greenberg, R. J. Electrochemical characterization of titanium nitride microelectrode arrays for charge-injection applications. In Proc. 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 1964–1967 (IEEE, 2003).
-
Deku, F. et al. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J. Neural Eng. 15, 016007 (2018).
-
Cui, X. T. & Zhou, D. D. Poly (3,4-ethylenedioxythiophene) for chronic neural stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 15, 502–508 (2007).
-
Jia, X. & Kohn, A. Gamma rhythms in the brain. PLoS Biol. 9, e1001045 (2011).
-
Opie, N. L. et al. Chronic impedance spectroscopy of an endovascular stent-electrode array. J. Neural Eng. 13, 046020 (2016).
-
McAdams, E. T., Lackermeier, A., McLaughlin, J. A., Macken, D. & Jossinet, J. The linear and non-linear electrical properties of the electrode–electrolyte interface. Biosens. Bioelectron. 10, 67–74 (1995).
-
Weiland, J. D. & Anderson, D. J. Chronic neural stimulation with thin-film, iridium oxide electrodes. IEEE Trans. Biomed. Eng. 47, 911–918 (2000).
-
Arcot Desai, S., Rolston, J. D., Guo, L. & Potter, S. M. Improving impedance of implantable microwire multi-electrode arrays by ultrasonic electroplating of durable platinum black. Front. Neuroeng. 3, 5 (2010).
-
Ludwig, K. A., Uram, J. D., Yang, J., Martin, D. C. & Kipke, D. R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly (3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 3, 59–70 (2006).
-
Mohit, A. A., Samii, A., Slimp, J. C., Grady, M. S. & Goodkin, R. Mechanical failure of the electrode wire in deep brain stimulation. Parkinsonism Relat. Disord. 10, 153–156 (2004).
-
Sankar, V. et al. Electrode impedance analysis of chronic tungsten microwire neural implants: understanding abiotic vs. biotic contributions. Front. Neuroeng. 7, 13 (2014).
-
Szarowski, D. H. et al. Brain responses to micro-machined silicon devices. Brain Res. 983, 23–35 (2003).
-
Hong, G. et al. Syringe injectable electronics: precise targeted delivery with quantitative input/output connectivity. Nano Lett. 15, 6979–6984 (2015).
-
Zhao, Z. et al. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00941-y (2022).
-
Viveros, R. D. et al. Advanced one- and two-dimensional mesh designs for injectable electronics. Nano Lett. 19, 4180–4187 (2019).
Acknowledgements
O.C. was supported by a National Science Foundation Graduate Research Fellowship. J.L.E. was supported by a Hertz Fellowship. M.M.M. is a Chan-Zuckerberg Biohub Investigator.
Author information
Authors and Affiliations
Contributions
M.M.M. and K.S. supervised the project. K.S., O.C., J.L.E. and D.K.P. performed literature review and wrote the manuscript. K.S. prepared the figures, with contributions from O.C. All authors contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.M.M. is an employee of iota Biosciences, Inc., a fully owned subsidiary of Astellas Pharma., Inc. D.K.P. is bound by a confidentiality agreement to not disclose details of a potential competing interest. K.S., O.C. and J.L.E. declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jacob Robinson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, K., Chen, O., Edmunds, J.L. et al. Translational opportunities and challenges of invasive electrodes for neural interfaces. Nat. Biomed. Eng (2023). https://ift.tt/mLNzkuc
-
Received:
-
Accepted:
-
Published:
-
DOI: https://ift.tt/mLNzkuc
"interface" - Google News
April 20, 2023 at 10:15PM
https://ift.tt/qIcQhgk
Translational opportunities and challenges of invasive electrodes for neural interfaces - Nature.com
"interface" - Google News
https://ift.tt/g5W20Df
https://ift.tt/9vA8jwW
Bagikan Berita Ini














0 Response to "Translational opportunities and challenges of invasive electrodes for neural interfaces - Nature.com"
Post a Comment